Abstract
Donor leukocyte infusion (DLI) can induce potent graft-versus-leukemia (GvL) activity in patients with relapsed acute leukemia after allogeneic hematopoietic stem cell transplant (HCT), but outcomes are generally poor, with high disease-related mortality. There is little data comparing logistics and outcomes of unrelated (uDLI) versus sibling (msDLI) DLI for relapse after allogeneic HCT. We performed a single-center retrospective cohort study to assess differences in time to obtain DLI from unrelated versus sibling donors, and to compare outcomes from both populations between 2000-2011.
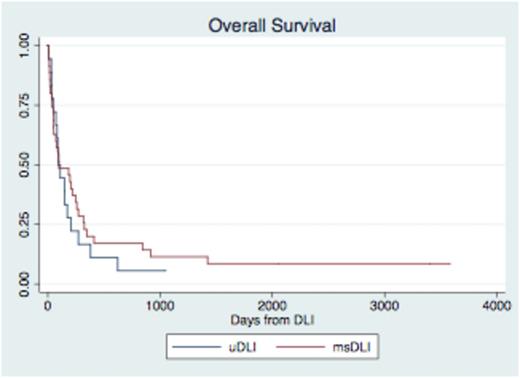
Fifty-three patients had relapsed AML, ALL, or high-risk MDS after allogeneic HCT from 2000-2011 at the University of Pennsylvania. Median time from relapse to infusion administration was 56 days for uDLI and 40 days for msDLI patients (p=0.034). However the time from DLI request to infusion was not significantly different between groups (27 days for uDLI and 40 days for msDLI, p=0.20) implying that the longer time from relapse to DLI was due to a longer time interval to request DLI from an URD. 29/35 msDLI patients and 10/18 uDLI patients received chemotherapy prior to DLI. Administration of chemotherapy did not significantly affect time from relapse to DLI (msDLI p=0.26, uDLI p=0.71). 15/35 (43%) of msDLI patients and 8/18 (44%) developed acute GVHD (p=0.91). There was no significant difference in development of IBMTR Grade C/D GVHD among uDLI (29%) and msDLI (24%) patients, p=0.38. The overall survival for the entire group was 96 days with no significant difference between recipients of uDLI and msDLI (p=0.49). Median time to death post DLI was 95 days for uDLI and 97 days for msDLI patients (see Figure I). Death was primarily from disease (74%). At 1 year after DLI, 7/35 (20%) recipients of msDLI were alive and 3/18 (17%) recipients of uDLI were alive (p=0.54). For those patients with response to DLI (defined as improved chimerism, morphologic response, or hematologic response), median time to progression was 68 days in uDLI recipients and 167 days in msDLI recipients (p=0.38).
For patients with relapsed acute leukemia and MDS after allogeneic HCT, the time from relapse to DLI was longer for recipients of uDLI compared to msDLI largely due to a longer time from relapse to DLI request. Once requested, there was no significant difference in time from request to infusion, implying this delay to not have an adverse impact on outcome of uDLI compared to MSDLI. Furthermore there was no difference in incidence of GVHD, relapse-free survival, or overall survival after uDLI versus msDLI. Outcomes unfortunately remain poor regardless of donor source. Our data support ongoing investigational efforts to improve outcomes for DLI recipients and overall supports feasibility of using both uDLI and msDLI in future studies.
p=0.49
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal