Abstract
Introduction: While the majority of patients (pts) with advanced stage DLBCL will be cured with R-CHOP, pts who fail front-line therapy continue to have a dismal outcome. Optimization of initial therapy remains an important goal. Interim PET scanning has been demonstrated to be prognostic in DLBCL and may be a promising tool to select pts in whom alternate non-cross-resistant therapies should be considered prior to the emergence of further drug resistance. With this rationale, a phase 2 trial was initiated to assess the feasibility and efficacy of PET-tailored therapy for DLBCL within the province of BC.
Methods: This phase II trial was conducted at multiple sites within the BC Cancer Agency. Patients >17 years of age with biopsy proven de novo advanced stage DLBCL, including PMBCL (stages 3 and 4, or stages 1 and 2 with B-symptoms or bulky mass > or = 10cm), with an ECOG PS <3 and no significant co-morbidities that would preclude administration of curative-intent R-CHOP were potentially eligible. Pts were treated with standard dose 3-weekly R-CHOP and could be enrolled at any time prior to cycle 4, provided they met all eligibility criteria and had routine staging investigations performed prior to treament. Staging PET scans were not required. Interim PET scans were performed at a centralized site following cycle 4 (between days 21-28 to minimize the risk of a falsely positive scan). PET scans were interpreted by a small core group of functional imaging radiologists according to International Harmonization Project criteria. Pts with a negative PET scan (PET-neg) received 2 additional cycles of R-CHOP, while pts with a positive PET scan (PET-pos) were switched to receive 4 cycles of R-ICE and underwent a final PET scan post-completion. As per policy in BC, pts with a positive PET post-treatment received consolidative radiation therapy (XRT) if feasible, and this was not considered to be an event with respect to progression-free survival (PFS). Pts with an indeterminate PET (PET-ind) were recommended to complete treatment with R-CHOP. Pts with disease progression during cycles 1-4 of R-CHOP were taken off study.
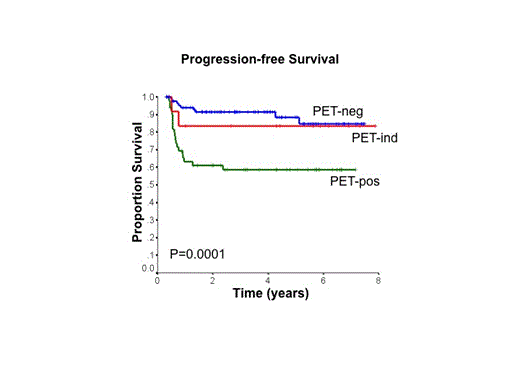
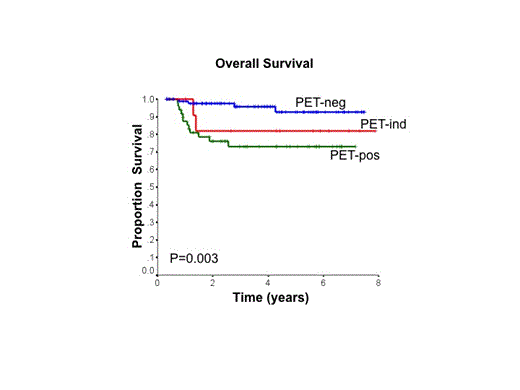
Results: 155 patients were enrolled between Oct 2006 and May 2014. Patient characteristics: median age 53 y (range 19-79); 54% male; 105 (68%) stages 3 or 4; 52 (34%) age >60 y; 55 (35%) ECOG PS >1; 90 (60%) elevated LDH; 44 (28%) extranodal sites >1; 81 (52%) bulky mass ³10cm; and 65 (42%) IPI score 3-5. 2 patients were non-evaluable: no interim PET due to toxicity (n=1), withdrawal (n=1). 3 pts were taken off study due to progression on R-CHOP. Of the 150 evaluable pts with interim PET, 88 (59%) were PET-neg, 50 (33%) were PET-pos and 12 (8%) were PET-ind. PET-pos pts were more likely to have elevated LDH and bulky disease at diagnosis. All PET-neg pts completed treatment with R-CHOP as intended and none received XRT. Of the 50 PET-pos pts, 2 refused to switch to R-ICE and completed treatment with R-CHOP (both received XRT). 48/50 PET-pos pts proceeded to R-ICE: 9 pts failed to complete all 4 cycles R-ICE due to toxicity and 6/9 switched back to R-CHOP; 3 progressed during R-ICE and did not have a final PET scan. The remaining 36/50 PET-pos pts completed 4 cycles R-ICE and underwent a final PET: 11/36 had a negative PET post R-ICE (2 received XRT, regardless); 25/36 had a positive PET post R-ICE (12 received XRT). Of the 12 PET-ind pts, 10 completed treatment with R-CHOP (1 with XRT), while 2 were switched to R-ICE (no XRT). With a median follow-up time of 45 mos, 4-y PFS and overall survival (OS) for the evaluable cohort were 79% and 87%, respectively. PET-neg pts had a very favorable outcome (4-y PFS 91%, 4-y OS 96%), whereas PET-pos pts had a less favorable outcome (4-y PFS 59%, 4-y OS 73%). PET-ind pts had an intermediate outcome with 4-y PFS 83% and 4-y OS 82%.
Conclusions: While PET-tailored therapy in pts with advanced stage DLBCL was feasible in BC, approximately 20% of patients were unable to tolerate the planned 4 cycles of R-ICE due to various toxicities, mainly myelosuppression. Pts who were PET-neg following 4 cycles of R-CHOP had an excellent outcome, without need for XRT. PET-pos pts had a poorer outcome, but did better than expected compared to historical reports. However, relatively few PET-pos pts converted to a negative PET scan following R-ICE, suggesting that simply switching to this alternate non-cross resistant chemotherapy regimen is insufficient to overcome the inherent resistance in this poor risk population.
Sehn:Roche/Genentech: Consultancy, Honoraria, Research Funding; Gilead: Consultancy, Honoraria; Lundbeck: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; Celgene: Consultancy, Honoraria; Seattle Genetics: Consultancy, Honoraria. Off Label Use: Use of R-ICE chemotherapy in front-line therapy of DLBCL.. Hardy:Roche Canada: Research Funding. Gill:Roche Canada: Research Funding. Al-Tourah:Roche Canada: Research Funding. Shustik:Roche Canada: Research Funding. Macpherson:Roche Canada: Research Funding. Yee:Roche Canada: Research Funding. Lam:Roche Canada: Research Funding. Savage:Roche Canada: Research Funding. Klasa:Roche Canada: Research Funding. Villa:Roche Canada: Research Funding. Gerrie:Roche Canada: Research Funding. Shenkier:Roche Canada: Research Funding. Slack:Roche Canada: Research Funding. Gascoyne:Roche Canada: Research Funding. Benard:Roche Canada: Research Funding. Wilson:Roche Canada: Research Funding. Tonseth:Roche Canada: Research Funding. Connors:Roche Canada: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal