Abstract
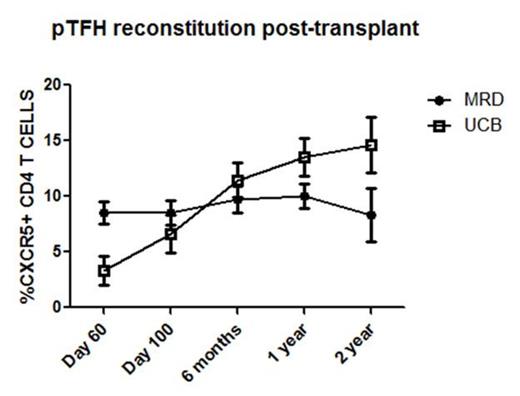
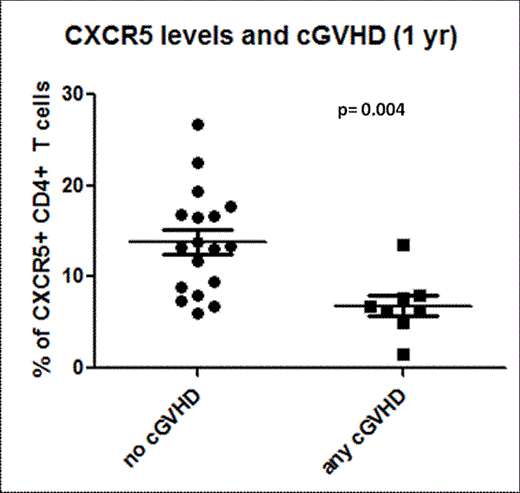
Allogeneic hematopoietic cell transplant (allo-HCT) results in profound immunodeficiency where patients remain susceptible to life-threatening infections for months to years. Although vaccination provides protection against viruses such as influenza, there is limited mechanistic evidence regarding the immune cell subsets directly involved. To better understand vaccination responses in allo-HCT recipients, a more specific analysis of the generation of influenza specific T cells is needed. Recently, it has been shown that a particular subset of CD4 T cells, T follicular helper (TFH) cells, play an important role in humoral responses to vaccination in humans. TFH cells express the receptor CXCR5, allowing them to traffic to secondary lymphoid tissues and provide help to B cells. We recently showed that TFH cells are also important in murine chronic graft-vs-host disease (cGVHD, Flynn and Blazar, Blood 2014), a process characterized by pathogenic B cell activity and chronic antibody deposition. Importantly, TFH cells and germinal centers were required for cGVHD and bronchiolitis obliterans in mice. Depletion of TFH effector cytokines (IL-21) or co-stimulatory ligands (ICOS and CD154) blocked germinal center formation and cGVHD. To date, no study has examined the recovery of TFH cells after allo-HCT and their association with vaccine specific responses or cGVHD. Here we investigated the reconstitution of peripheral T follicular helper cells (pTFH, CD3+CD4+CXCR5+ cells) in patients undergoing UCB (n=14) or matched sibling PBSC transplantation (n=12). Notably, UCB recipients had lower percentages of pTFH cells at day 60 following transplant compared to PBSC MRDs (2.0% ± 0.2 vs 8.6% ± 1.0, p<0.001). This finding is likely explained by the graft content of pTFH cells which is higher in MRD donors.UCB grafts contain no CXCR5+ CD4 T cells. Conversely, the pTFH cells in UCB recipients recovered to a higher level than PBSC MRD recipients at >1 year following transplant (13.84% ± 1.4 vs 9.7% ± 1.0 SEM, p=0.019). Comparing the trajectory of pTFH recovery, MRD recipients remain stable over time whereas UCB donors show a progressive rise (Figure 1). Also, in both UCB and MRD transplants, pTFH cells are mainly composed of central memory cells (CD27+/CD45RO+; 82.17% ± 2.588 vs 87.44% ± 1.664, p=0.1664), demonstrating that circulating pTFH cells are antigen experienced. Strikingly, when patients with any cGVHD (n=8) from both groups were combined and analyzed at 1 year, there were significantly lower percentages of pTFH cells compared to those without cGVHD (n=18) (6.9% ± 1.2 vs. 13.8% ± 1.3, p=0.004) (Figure 2).
Lastly, we have previously demonstrated that donor source (MRD PBSC vs UCB) and time from transplant (<1 yr and >1 yr post-allo-HCT) were associated with influenza vaccine responses. To study the role of pTFH cells in response to influenza vaccination, we analyzed samples of patients on a randomized controlled trial receiving 1 versus 2 doses of influenza vaccine following transplant from matched related or UCB donors. Blood samples were drawn pre-vaccination, then at 4 weeks patients were randomized to receive another dose of vaccine or not. Samples from each group were also collected at 8 weeks. We then evaluated polyfunctional, influenza-specific pTFH cells (expressing CD3, CD4, CXCR5, CD154) producing one, two, three or four cytokines (eg IFNy, TNFa, IL-17 and IL-2) in response to influenza peptide. Notably, these antigen-specific pTFH cells expanded with varying responses to 1 or 2 doses of influenza vaccine in vivo. These data provide biologic evidence of an increasing frequency of antigen-specific CD4 T cells following a second dose of vaccine. In combination with our data showing a correlation between absolute B cell numbers and vaccine responders (4x Ab response p=0.03, ELIspot IFNy response p<0.01), these data also support the hypothesis that vaccine responses post-HCT are multi-factorial (eg need T cell help and a functional B cell compartment).
These studies are the first to evaluate the recovery of pTFH cells following allo-HCT. Our data demonstrate significant differences in the proportion of pTFH cells following allo-HCT based on stem cell source and the presence of cGVHD. They also show that antigen specific pTFH cells can be detected after influenza vaccination and provide additional evidence for some of the differences in vaccine responses.
Miller:Coronado: Speakers Bureau; BioSciences: Membership on an entity's Board of Directors or advisory committees; SAB: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal