Abstract
Acknowledgments: This study was supported by the grants from the National Natural Science Foundation of China (Grant No. 81270644 and 81230013), the Major State Basic Research Development Program of China (973 Program No. 2013CB733700), doctoral funding from The Ministry of Education of China (grant no. 20110001110039) and collaborative innovation center of Hematology, China. The authors thank all of the core facilities at the Peking University Institute of Hematology for sample collection.
Background: There were good evidences that natural killer cells and T regulatory cells, which were expanded and stimulated by the application of interleukin-2 (IL-2). It was found that low dose of IL-2 administration was associated with preferential, sustained Treg cell expansion in vivo and amelioration of the manifestations of chronic graft-versus-host disease (GVHD).The aim of this randomized study were to confirm that low dose of IL-2 early application post transplantation can prevent chronic GvHD occurrence following allogeneic hematopoietic stem cell transplantation (all-HSCT) in patients with standard risk acute leukemia.
Methods: This was a single-centre, prospective, open-label, randomized control trial. Standard risk leukemia patients over 15 years old (except Ph+ ALL and T-ALL) undergone unmanipulated blood and marrow allo-HSCT following day 60 post-transplantation were randomly assigned (in a 1:1 ratio, by a computer-generated randomisation list) into treated group (with low dose IL-2 treatment) or controlled group (without any intervention post-transplantation). Interleukin-2 treatment (daily 1¡Á106IU per square meter of body-surface area) repeats every 14 days for 4 to 6 courses in the absence of disease progression or unacceptable toxicity. Blood tests were performed before IL2 treatment and weekly for the first two weeks and then every other week until the completion of 6 course therapy to monitor the immunologic impact of ultra-low dose subcutaneous IL-2 in patients after transplantation.
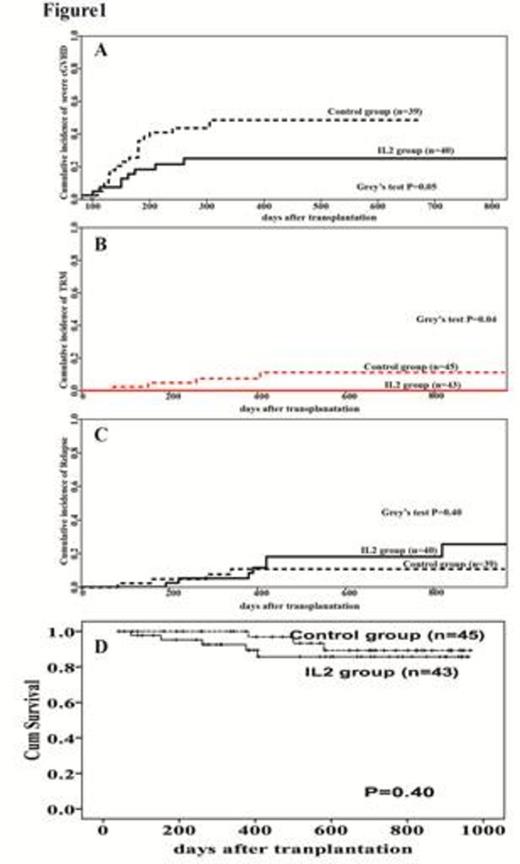
Results: This study begins at Jan 2012, and 360 patients were estimated to enroll. Interim analyses were planned post near 25% of total numbers of patients¡¯ enrollment. Until July16th, 2014, there were 88 patients (43 patients in test group and 45 patients in control group) were randomized in this trial. The follow-up of 79 patients exceeded 180 days. Interim analysis showed that the cumulative incidence of sever chronic GVHD in IL2 group were significantly lower than those in control group (P=0.05, Figure 1A), meanwhile, transplantation related mortality (TRM) were also lower in IL2 group compared to those in control group (P=0.04, Figure 1B). No significantly differences were found in cumulative incidence of relapse between IL2 group and control group. The overall survival of two groups was comparable. Low dose of IL-2 in vivo application augmented reconstituted CD4+CD25highCD127-/low T regulatory cells and natural killer cells. Meanwhile, cytotoxicity of NK cells in IL2 group was significantly higher than the control group during the IL2 treatment period. IL2 therapy has no effect on reconstitution of absolute number of CD3+T cells, CD4+ T cells, CD8+ T cells, as well as conventional T cells, Th1 cells, Th17 cells reconstitution.
Conclusion: Low dose of IL2 could expand CD4+CD25highCD127-/lowT regulatory cells and natural killer cells and prevent chronic graft-versus-host disease. This trial is registered with ClinicalTrials.gov, number NCT01517347.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal