Abstract
Background: Delayed hematopoietic recovery after myeloablative cord blood transplantation (CBT) is associated with higher infection-related morbidity and mortality when compared to recipients of conventional donor grafts. In particular, prolonged neutropenia, related to low cell doses provided by the cord blood (CB) graft, results in higher rates of infection in the first 100 days post-transplant. To overcome this issue, our group has explored ex vivo expansion of CB-derived CD34+ cells in the presence of Notch ligand. We have previously demonstrated that infusion of this expanded progenitor cell product leads to a significant reduction in the time to neutrophil engraftment in the myeloablative CBT setting [Delaney C, et al, Nat Med 2010]. We now hypothesize that faster neutrophil engraftment is associated with lower risk of bloodstream infections in the first 100 days post-transplant in patients receiving ex-vivo expanded progenitors (EXP) compared to patients receiving conventional CBT (CON).
Methods: Between April 2006 and June 2014, a total of 93 consecutive patients were enrolled on multicenter studies coordinated by our Institution. Of these 93, 55 (59%) received single (n=9) or double (n=46) unmanipulated CB graft, while 38 (41%) patients received 1 or 2 unmanipulated CB units plus infusion of either a fresh partially HLA-matched (n=23) or a cryopreserved non-HLA matched (n=15) ex-vivo expanded CB unit. All patients received the same conditioning regimen of Fludarabine (75mg/m2), Cytoxan (120mg/kg) and TBI (13.2 Gy) over 8 days. GVHD prophylaxis consisted of cyclosporine and mycophenolate mofetil. Cox regression model was used to assess the risk of bacterial infection where "failure" was defined as the first occurrence of bloodstream infection within 100 days following CBT.
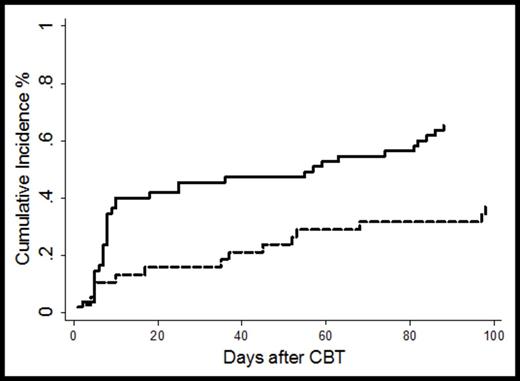
Results: The median patient age was 22 years (range 0.6-45) and 26 (range 3-45) for CON and EXP group respectively (p=0.51); gender, race and CMV serostatus were not different between the 2 groups. Disease distribution was similar in the two groups, with most patients undergoing CBT for AML (n=47), ALL (n=28) and MDS (n=10). Median pre-thaw TNC/kg x107 was 4.97 (range 3.4-16.6) and 11.2 (range 2.7-30.4) for the CON and EXP groups respectively, and pre-thaw CD34 cells/kg x106 was 0.19 for the CON (range 0.04-0.98) and 6.3 for the EXP group (range 0.12-49.7). As expected, time to engraftment was significantly faster for patients receiving the EXP units at 17 days (range 6-41) vs. 25 days in the CON group (range 14-45, p<0.01). Only 1 case of graft failure was observed in the EXP group. The median time to first positive bloodstream infection in the EXP cohort was 36 days (range 3-98) vs. 8 days (range 1-88) in the CON group. The cumulative incidence of bacteremia was significantly lower in the EXP group (n=14) compared to the CON group (n=36) at 37% vs. 65% respectively (p=0.005, Figure 1). The hazard ratio (HR) of bacteremia episodes was significantly lower among patients receiving the expanded cells (HR 0.43; 95% CI 0.23-0.79; p=0.007). Among first documented bacteremic episodes, 5 of 50 (10%) were gram-negative rod bacteremias, all of which were in the CON group.
Conclusions: Infections remain a significant risk for morbidity and mortality after myeloablative CBT and are the leading cause of early treatment related mortality. Our data suggest that there was a significant reduction in risk of bacteremia in the first 100 days post myeloablative CBT and a longer time to first infection when patients received ex vivo expanded progenitor cells, either as a partially matched fresh infusion or as an off-the-shelf, non-HLA matched expanded product. Infusion of the expanded cells resulted in reduced time to myeloid engraftment and offered a protective effect against episodes of bacteremia, but the exact mechanisms are presently unknown. It is possible that the expanded cells may home to regions of mucosal injury post TBI and mitigate breakdown thus reducing bacterial translocation. Further research to further define the expanded cells' mechanism of action with regards to infection reduction is currently underway in animal models.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal