Abstract
Novel therapeutic approaches are urgently needed for many malignancies such as Acute Myeloid Leukemia (AML). We have developed a new therapeutic strategy based upon NK cell immunotherapy that exhibits high clinical potential based upon cell and animal studies. While the harnessing of NK cells for cellular therapy against malignancies has been a topic of interest for several decades, our approach overcomes a major hurdle of insufficient NK cell cytotoxic activity. We have identified that targeting the kinase GSK3 through pharmacologic and genetic approaches leads to the hyperactivation of human blood derived NK cells and a significant improvement in efficacy as compared to traditionally used activated NK cells or chemotherapy in our mouse AML model systems. Importantly this GSK3 inhibition can be achieved through a short ex-vivo incubation of NK cells with a GSK3 inhibitor paving the way for a rapid implementation into a clinical trial. Utilizing both in vitro studies with AML cell lines (ex. OCI-AML3 and HL-60)) and primary human AML cells we observe approximately a 50% increase in efficacy with GSK3 inhibited NK cells as compared to untreated NK cells. Further, we demonstrate significant efficacy of GSK3 inhibited NK cells in a mouse model of circulating human AML. After 4 weekly injections of human NK cells, there is a 50% greater reduction in human AML cells present in the mouse bone marrow with GSK3 inhibited NK cells as compared to vehicle treated NK cells.
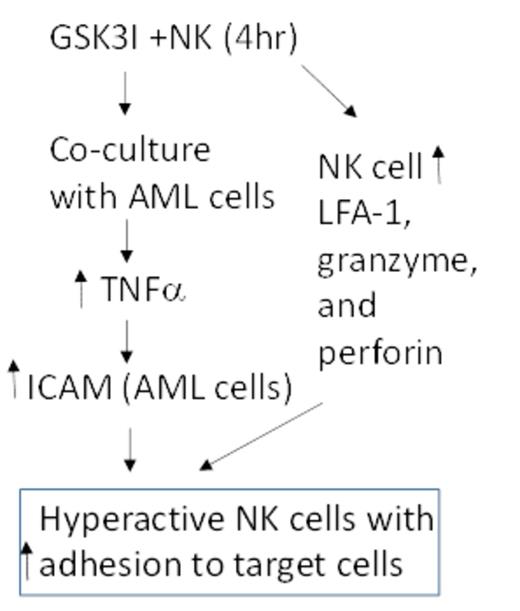
Besides efficacy studies, our work has led a model of how GSK3-inhibition enhances NK cell activity as depicted in figure 1. GSK3 inhibition leads to a dramatic increase in adhesion of NK cells to target cells as demonstrated by a flow cytometric adhesion assay (49% vs 83% after 20 min incubation) as well as live cell imaging. Consistent with the increased adhesion, GSK3 inhibited NK cells as well as target cells (after co-incubation) exhibit increased expression of essential NK cell-target adhesion molecules including L-selectin (on NK cells) and ICAM (on target cells). The induction of ICAM on target cells is due to a marked induction in TNFa production from the NK cells upon incubation with target cells (>7 fold increase in TNFa production). TNFa neutralization impairs the NK activity of the GSK3 inhibited NK cells (~30%) but not vehicle treated cells. Finally, GSK3 inhibition also leads to changes in the NK cells that enhance activity such as increased expression of granzyme and perforin and secretion of IFNg. Overall, our work has a revealed a novel strategy for NK cell therapy that holds high clinical potential.
Model of how GSK3 inhibition leads to hyperactive NK cells. GSK3I - GSK3 inhibitor
Model of how GSK3 inhibition leads to hyperactive NK cells. GSK3I - GSK3 inhibitor
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal