Abstract
The success of allogeneic hematopoietic stem cell transplantation (allo-HSCT) is limited by acute graft-versus-host disease (GVHD). Improving the procedure depends on identifying the mechanisms that contribute to this damaging T cell reactivity, while preserving graft-versus-leukemia (GVL) activity against hematopoietic malignancies.
"Tonic" type I IFN signaling in BMT recipients and therapeutic application of recombinant IFN-α have been shown to play an important role in defining the balance between GVHD and GVL responses, but the molecular mechanisms inducing this protective response remains unknown. In this regard, pattern recognition receptors (PRRs) that detect cytosolic nucleic acids and lead to the production of large amounts of type I interferons (IFN-α/β) such as the family of RIG-I-like receptors (RLRs) are of particular interest. RLRs, a subfamily of the cytoplasmic DExD/H- box family of helicases, consist of three members: retinoic acid inducible gene I (RIG-I), melanoma differentiation factor 5 (MDA5), and laboratory of genetics and physiology 2 (LGP2). RIG-I senses viral and bacterial RNA to induce the production of type I interferons, proinflammatory cytokines and inflammasome activation. Double-stranded RNA (dsRNA) carrying a 5'-triphosphate (3pRNA) has been identified as the natural ligand for RIG-I and serves as a selective trigger for RIG-I signaling. Although initially characterized as a main regulator for antiviral host defense, mice deficient in components of the RLR and type I IFN signaling pathway develop inflammatory bowel disease (IBD)-related pathologies. Furthermore, patients suffering from IBD show a highly significant downregulation of RIG-I in ileal epithelial cells and in a recent meta-analysis of genome-wide association studies (GWAS) data, IFNAR1 and MDA-5 were identified as primary candidate genes in susceptible loci for IBD. Together, these results indicate that RLRs and type I IFN signaling have important functions in the suppression of IBD by yet ill defined mechanisms. Given that the pathophysiology of GVHD shares several features with inflammatory bowel disease (IBD), we tested the role of the RIG-I pathway in the context of allo-HSCT.
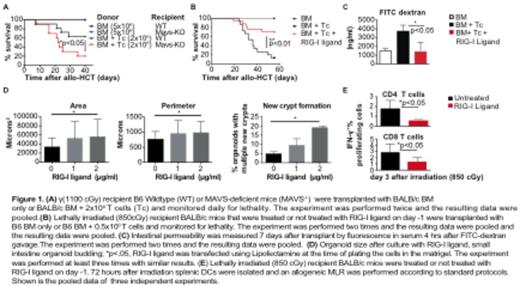
We utilized MAVS-deficient mice, which lack a common adapter for RIG-I signaling, as recipients in an MHC-disparate (BALB/c into B6) model of allo-HSCT. Compared to wild-type (WT) B6 mice, MAVS-KO mice receiving allogeneic BM + T cells displayed significantly worse GVHD mortality (Fig.1 A). Given the enhanced GVHD observed in the absence of RIG-I signaling, we hypothesized that selective engagement of RIG-I by 3pRNA (RIG-I ligand) in vivo would protect recipients from GVHD. Using a B6 into BALB/c model we observed that i.v. administration of a selective RIG-I ligand on d-1 significantly reduced mortality, weight loss and intestinal GVHD histopathology (Fig. 1B and data not shown). In addition, the translocation of LPS and microorganisms from the bowel lumen through the damaged intestinal mucosa to the systemic circulation during pre-transplant conditioning represents a crucial step in GVHD pathophysiology. We observed that administration of RIG-I ligand prior to allo-HSCT augmented intestinal barrier function measured by less fluorescence in the serum after FITC-dextran gavage compared to untreated WT recipients (Fig. 1C). Moreover, RIG-I stimulation augmented epithelial regeneration as determined by organoid formation from freshly isolated crypts (Fig. 1D) and inhibited activation of dendritic cells (DCs) during pre-transplant conditioning (Fig. 1E). To investigate the impact of 3pRNA administration during GVT, we used luciferase+ A20 bioluminescence in B6 into BALB/c tumor challenge recipients demonstrating that RIG-I ligands do not limit GVL (data not shown). Taken together, our results (i) uncover a previously unknown role of the RIG-I-MAVS signaling pathway in GVHD and (ii) offer a novel strategy to foster epithelial regeneration and inhibit antigen presentation during pre-transplant conditioning, while maintaining GVL.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal