Abstract
Promising results from murine models and early stage clinical trials have shown that adoptive transfer of regulatory T cells (Treg) prevents graft-versus-host disease (GvHD). However, the primary target of Treg mediated protection against GvHD is yet to be fully defined. We have previously shown that the presence of Treg during effector T cell priming is able to ameliorate cutaneous GvH reactions in vitro by blocking effector cell migration. This has led to the hypothesis that Treg modulation of dendritic cells (DC) could be a key mechanism by which Treg exert their protective role in GvHD. DC are fundamental for the initiation of allo-reactive immune responses and are critical in GvHD pathogenesis. We investigated the effect of Treg on the phenotypic profile and allo-reactive functions of DCs. Furthermore, the impact of Treg treatment on the ability of DCs to induce GvH target tissue damage was examined for the first time using an in vitro human GvHD skin explant model.
Immature, mature and Treg treated DCs were generated from immuno-magnetic isolated monocytes (im-DC, mat-DC and Treg-DC respectively). The three moDC populations were generated using the well-established 6 day culture with GM-CSF and IL-4 followed by 24 hour LPS maturation. Treg were added on day 3 of moDC culture. Im-DC, mat-DC and Treg-DC were harvested on day 7 and tested in parallel as stated below. Prior to functional assays Treg-moDC were isolated by FACS sorting via FSC/SSC/CD3neg gating to remove Treg present in the co-culture.
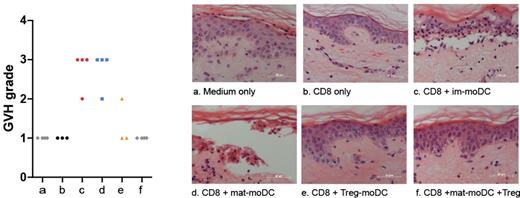
Our results revealed that Treg-DC displayed a semi-mature phenotype with CD83, CD80 and CD86 expression significantly lower than mat-moDC (p<0.005) but significantly higher than im-moDC (P<0.05) whilst HLA-DR levels were comparable to mat-moDC but significantly higher than imDC (p<0.005). Treg-DC also expressed CCR7 comparable to that of im-DC but markedly higher than mat-DC (p=0.052). Distinct morphology of Treg-DC, defined by Giemsa staining, corroborated their semi-mature status. Data from FITC-dextran uptake showed a significant reduction in antigen-capture capacity of Treg treated im-DC compared to untreated im-DC (p=0.047). The Treg mediated functional impairment of DCs was also associated with significantly higher expression of LAP-TGFβ1 on Treg-DC when compared to that of mat-DC (P<0.05). Treg-DC were also markedly defective in stimulating activation and proliferation of allo-reactive CD8 T cells, detected by CD25 expression and CFSE dilution respectively (p=0.009, p=0.046). Interestingly the presence of Treg throughout the entire allo-response induction resulted in a more potent reduction in activation and proliferation than when only the DC were treated with Treg (p=0.009, p=0.0085). Furthermore, allo-reactive CD8 T cells primed with Treg treated moDC were less able to mediate cutaneous GvH tissue damage (Figure 1).
In conclusion, attenuation of DC signature and function is key for Treg mediated protection against GvHD. However, isolated DC modulation by Treg was less effective in suppressing CD8 T cell allo-responses compared to the continued presence of Treg during the CD8 T cell priming, activation and proliferation, suggesting that Treg exert most effective function via multidimensional modulation on the DC, T cells and DC-T cell interactions simultaneously.
Skin histopathology induced by CD8 T cells primed with im-DC, mat-DC and Treg-DC
Skin histopathology induced by CD8 T cells primed with im-DC, mat-DC and Treg-DC
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal