Abstract
Introduction. Intra-Osseous (IO) transplantation has been proposed as a strategy to enhance human UCB (umbilical cord blood; hUCB) engraftment in NOD SCID-gamma (Nude Obesity Diabetic Severe Combined Immunodeficient, NSG) mice. Human Mesenchymal Stromal Cells (hMSC) have been shown to support the growth and differentiation of hematopoietic stem cells. We hypothesize that IO co-transplantation of hMSC and CD34+ selected hUCB may be effective in improving early engraftment in a NOD/SCID-gamma (NSG) mouse model. This concept would provide the basis for postulating that IO co-transplantation of hUCB and hMSC may improve engraftment in patients undergoing umbilical cord transplant.
Methods. Umbilical cord grafts were obtained from our cord blood procurement program at University Hospitals Case Medical Center under an IRB approved protocol. CD34+ cells were labeled using the Miltenyi CD34+ Microbead Kit (Miltenyi Biotec, Bergiiisch Gladbach, Germany) and magnetically separated using the Miltenyi MACS system. CD34+ cells recovered from the column were re-suspended to the appropriate concentration per cohort and infused fresh.
Human MSC (CD105+ CD73+ CD45- CD14-) were obtained from bone marrow donors using Percoll gradient isolation and culture-expanded to a homogeneous population under approved protocols in the Cellular Therapy Laboratory. MSCs were preserved in DMSO and when needed were thawed and re-suspended at the appropriate concentration per cohort.
We used a NSG mouse (Jackson Laboratories) model of transplant to compare the rates of engraftment amongst 6 mouse cohorts by examining bone marrow and peripheral blood cellular composition at 6 weeks following transplant. Following non-lethal irradiation (300rads), 6 groups of NSG mice (5 mice per cohort) were studied: 1) intravenous (IV) 5x105 hUCB CD34+ cells, 2) IV 5x105 hUBC CD34+ cells and 1x106 hMSC, 3) IO 5x105 hUCB CD34+ cells, 4) IO 5x105 hUCB CD34+ cells and IO 1x106 hMSC, 5) IO 5x105 hUCB CD34+ cells and IV 1x106 hMSC, and 6) IV 5x105 hUCB CD34+ and IO 1x106 hMSC. hMSC dose was arbitrarily set at 2:1 with hUCB CD34+ cell dose. IV injections: cells were administered via tail vein injection suspended in a total volume of 200ul. IO injections: cells were administered via bilateral tibia IO injections suspended in a total volume of 40ul. At 6 weeks, the mice were sacrificed and their peripheral blood and bone marrow were analyzed for human hematopoietic cells using antibodies against human CD45, CD3, CD13, CD14 and CD19 (Becton Dickinson Biosciences). Human engraftment was expressed as percentage of CD45+ cells compared to total bone marrow cells.
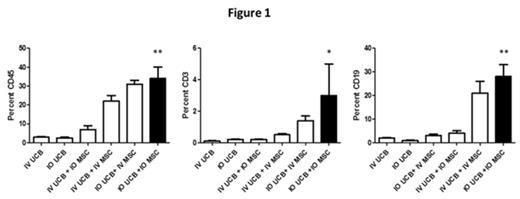
Results. All cohorts showed evidence of human hematopoietic engraftment. Analysis of human CD45, CD3 and CD19 revealed the highest level of engraftment in the IO hUCB /IO hMSC cohort, which was significantly improved over the IV hUCB cohort (Table 1, Percent human CD45, CD3 and CD19 in the right tibial bone marrow at 6 weeks, p values as compared to IV hUCB cohort). The right tibial bone marrow analysis of human CD13 and CD14 revealed no significant difference between cohorts.
We observed that IO co-transplantation of hMSC and hUCB led to superior engraftment of CD45, CD3 and CD19 lineage cells in the bone marrow at 6 weeks as compared with the IV hUCB cohort controls (Figure 1, human CD45, CD3 and CD19 analysis of right tibial bone marrow at 6 weeks; * = p <0.05, ** = p<0.001). The frequency of human hematopoietic cells within the peripheral blood was not statistically different among various cohorts at 6 weeks.
Conclusions. This study suggests that intra-osseous co-transplantation of hMSC and hUCB may improve early engraftment in NSG mice. The hypothesis that this strategy may improve engraftment of single unit CB transplantation in humans in intriguing and will be investigated in a phase I clinical trial.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal