Abstract
BACKGROUND
Cytotoxic treatment in B-cell precursor acute lymphoblastic leukemia (BCP-ALL) patients induces a dramatic decrease in B-cell precursor (BCP) and mature B-cell numbers, followed by regeneration of BCPs in the bone marrow (BM) and subsequent replenishment of mature B-cells in the peripheral blood (PB) in between treatment blocks and after stop of treatment. To understand the degree of B-cell recovery after such dramatic changes, we first evaluated the composition of the B-cell population in the BM and PB of pediatric BCP-ALL patients during and after therapy. Secondly, we investigated whether the immunophenotypic maturation of BCPs in regenerating BM is similar to normal BCP development or whether such regeneration induces immunophenotypic aberrancies, which could potentially hamper minimal residual disease (MRD) detection. Finally, we assessed whether compensatory proliferation plays a role during B-cell regeneration, since enhanced proliferation might limit the B-cell receptor diversity and consequently may affect susceptibility to infections during and after therapy.
METHODS
For immunophenotypic characterization of different B-cell subsets, 8-color flow cytometry was performed on fresh BM and PB samples at different time points after start of therapy (DCOG ALL11-protocol). To study BCP maturation, a multidimensional maturation pathway based on 5 backbone markers was designed and the expression pattern of several differentiation markers during this maturation pathway was evaluated. To assess proliferation in BCP subsets, BM samples were stained with subset-specific antibodies and DRAQ5 for cell cycle analysis. The proliferation history of sorted pre-B-II-small and immature subsets in BM and sorted mature B-cell subsets in PB was assessed by performing a kappa-deleting recombination excision circle (KREC)-assay.
RESULTS
BCP regeneration occurred mainly at day 78, month 5 and after stop of therapy. The BCP compartment in regenerating BM at time points during therapy showed a shift towards the most immature stages. In PB, mature B-cell numbers decreased after start of therapy and newly generated mature B-cells subsets reappeared at month 5 and after stop of therapy.
Importantly, the BCP maturation pathway with its expression patterns of CD10, CD34, CD58, CD66c, CD38, CD123, CD9, CD81, CD24, TdT, Igκ and Igλ was comparable between regenerating BM and BM of healthy individuals, albeit that a shift in the relative BCP subset distribution was observed in regenerating BM.
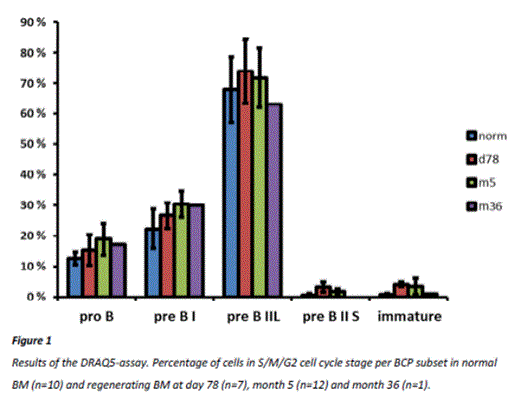
As expected, most proliferation in BM of healthy controls occurred in the pre-B-II-large subset (68% ±11% (mean ±SD) proliferating cells). Comparable percentages of proliferating pre-B-II-large cells were found in regenerating BM: 74%±10% at day 78, 72%±10% at month 5 and 63% (preliminary data, n=1) at one year after stop of therapy (month 36). Also pre-B-I cells showed some proliferation, with no significant differences between normal and regenerating BM (Figure 1). If present, the pre-B-II-small and immature BCP subsets showed no proliferation in regenerating and normal BM. KREC-analysis of sorted pre-B-II-small and immature subsets confirmed that no cell divisions had occurred after IGK-rearrangement in normal BM as well as regenerating BM at month 5 and month 36. Low numbers of pre-B-II-small and immature cells precluded KREC-analysis at day 78.
KREC-analysis of the various mature B-cell subsets in PB showed no significant difference in proliferation history between PB of patients at different time points during or after therapy and PB of healthy controls.
CONCLUSIONS
In BCP-ALL patients, the B-cell compartment is drastically affected during treatment. Subsequent regeneration of BCPs and mature B-cells occurs at different time points during therapy and after stop of therapy. Immunophenotypically, BCP maturation in regenerating BM is similar to normal B-cell differentiation, indicating that MRD detection will not be hampered by aberrant immunophenotypes of regenerating BCPs. Importantly, no enhanced proliferation is observed in BCP subsets in BM and mature B-cells subsets in PB of patients during and after therapy. The lack of compensatory proliferation suggests that B-cell regeneration is due to a larger influx of non-committed stem cells into the B-cell lineage and indicates that a diverse immune repertoire will most likely be restored during recovery of the B-cell compartment.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal