Abstract
Background Although AML and ALL are thought to arise from different progenitor cells, (common myeloid vs. common lymphoid) they share many clinical features and pathophysiological characteristics, such as excessive proliferation in association with blocks in differentiation, that raise the question of whether they might share mechanistic commonalities despite separate ontologic origins. This in turn would suggest common therapies to utilize in cases with similar mechanisms of action. Using reverse-phase protein lysate arrays (RPPA), we strove to determine if RPPA could 1) determine protein activation differences between pediatric ALL and AML, and 2) determine if protein expression clusters were affected by sample collection method, an issue critical to assessing protein activation pathways in the setting of cooperative group clinical trials.
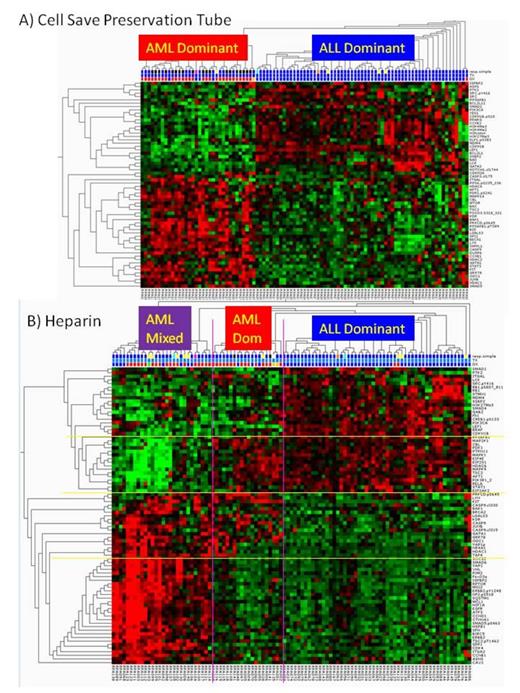
Methods We generated a custom RPPA with 132 leukemia enriched samples. The array included 59 AML (3 APL, 1 TMD, 1 relapse) and 73 ALL (58 pre-B, 15 T-ALL, 6 relapsed) patients. Median age was 9.1 years (range 0.0 – 22.1 years). Samples were enriched for leukemia cells using ficoll followed by magnetic bead separation, either CD3/CD19 depletion (AML), or B/T-cell isolation (ALL). The RPPA was probed with 194 strictly validated antibodies (139 antibodies assessing total protein expression, 36 phosphoproteins, 6 cleaved forms, and 3 methylation sites). Samples were collected in either heparin tubes (n=101) or CellSave preservations tubes (Veridex) (n=97), with 57 samples having both tube types.
Results Clustering demonstrated that AML and ALL have highly different protein expression and activation patterns. Pre-treatment samples collected in Cell Save preservation tubes revealed two protein clusters dividing the patients into two groups based on the levels of 62/194 significantly differentially expressed proteins (p=0.01, FDR = 0.027). An AML-dominant cluster contained 31 AML and 4 ALL among its 35 members. The ALL-dominant cluster had 62 ALL and a lone AML sample among 63 members. Using heparin tubes, four protein clusters were observed which divided patients into three clusters based on the differential expression of 83 proteins (p=0.01, FDR<0.023). The ALL-dominant cluster, composed of 53 members, had 51 ALL and 2 AML samples. The two AML patients in the ALL group included a patient with difficult to treat AML that achieved CR after 3 cycles of standard AML therapy with the proteasome inhibitor bortezomib, and a patient with 11q23 ALL that subsequently underwent a lineage switch to AML. There were two AML clusters, one with 85% AML (middle cluster in figure) with 17 AML and 3 ALL and the other with 27 patents (far left cluster) consisting of 70% AML (18 AML and 1 TMD) along with 8 ALL samples. In the far left cluster, of the 8 ALL cases, 5 had changes involving chromosome 21, including four with t(12;21), and 1 with trisomy 21. ALL samples in total had only 8/73 with the t(12;21) translocation, indicating a statistically significant difference (p=0.002, two-tailed Fisher's exact test).
Twenty-seven proteins were different in both the CellSave (CS) and heparin analyses. An additional 34 proteins, mostly epigenetic modifiers and apoptosis pathway proteins (Bcl2, BAD, BAX), were seen in the CS samples. An additional 52 proteins were detected in the heparin group, many related to cell stress pathways.
Conclusion Protein expression profiles strongly divided pediatric ALL from AML. Since this classification scheme is dependent on protein expression and functional activation states, it may be possible to further identify patients with different risk characteristics based on protein expression profiles. However, at least 111 of 194 proteins showed similar expression in both AML and ALL suggesting that there may be commonalities in mechanisms shared between leukemias of different lineages. Further analysis of protein functional groups and pathway utilization are in progress to define both differences and commonalities.
Off Label Use: bortezomib: off label use in pediatric AML as part of a COG clinical trial.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal