Abstract
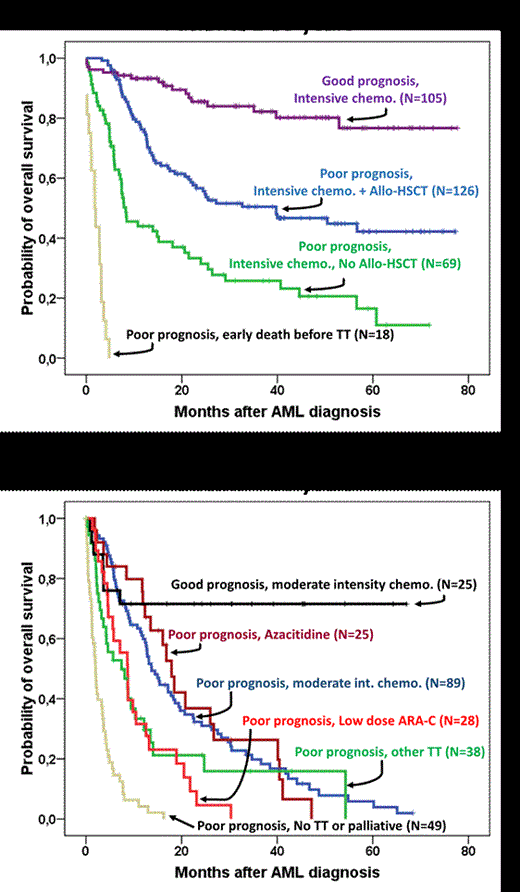
We evaluated in this study the therapeutic management of all newly diagnosed acute myeloid leukemia (AML) patients followed in our center between July 2007 and September 2013, after stratification on age, prognostic factors, whether they were candidate to allogeneic hematopoietic stem cell transplantation (allo-HSCT), transplanted or not with the evaluation of each therapeutic strategy and its impact on overall survival. A total of 572 consecutive newly diagnosed AML patients were included; there were 311 (54%) males and 261 females with a median age of 63 years (range: 20-92), 406 (71%) were de novo AML and 166 (29%) secondary AML. Complete cytogenetic and molecular biology data were collected for all patients and prognosis was differentiated according to the European LeukemiaNet classification (Dohner et al. Blood 2010). Accordingly, 335 (59%) patients were unfavorable, 83 (15%) favorable, 48 (8%) intermediate I and 106 (18%) were in intermediate II category. Following the Acute Leukemia French Association (ALFA) guidelines for allo-HSCT in AML, patients with intermediate II and unfavorable prognosis ≤ 65 years should receive intensive chemotherapy followed by allo-HSCT in the presence of related or unrelated HSCT donors. We divided the population into two sub-populations, the first was considered as young with age ≤ 65 years (N=318, 56%) and the second considered as old with age > 65 years (N=254, 44%). In the young population, there was 4 groups, group 1: patients with good prognosis (favorable/intermediate1) who received intensive chemotherapy within or according to ALFA protocols (N=105, median age= 47 years); group 2: patients with poor prognosis (intermediate2 /unfavorable) who received intensive chemotherapy within or according to ALFA protocols followed by allo-HSCT (N=126, median age= 50 years), group 3: patients with poor prognosis who received only intensive chemotherapy without allo-HSCT (N=69, median age= 57 years), and group 4: patients with poor prognosis who could not be treated due to early death (N=18). In the old population we distinguished, group 1: patients with good prognosis who received moderate intensity chemotherapy within or according to ALFA protocols (N=25, median age=73 years); group 2: patients with poor prognosis who received azacitidine (N=25, median age=76 years), group 3: patients with poor prognosis who received moderate intensity chemotherapy (N=89, median age=76 years), group 4: patients with poor prognosis who received low dose Ara-C (N=28, median age=76 years), group 5: patients with poor prognosis who received other treatment (N=38, median age=77 years) and finally group 6: patients with poor prognosis considered as palliative or who did not receive any treatment (N=49, median age=77 years). After a median follow-up of 34 months (range: 4-77) for surviving patients, the 2-years probability of overall survival (OS) in the young population for groups 1,2,3 and 4 was 84%, 56%, 31% and 0% respectively; and in the old group it was 71%, 37%, 31%, 5%, 21% and 0% for groups 1,2,3,4,5 and 6 respectively (figure).
We showed that newly diagnosed AML patients with good prognosis all ages included could achieve very good survival rates after intensive chemotherapy; allo-HSCT after induction chemotherapy remains the best therapeutic option for fit patients with poor prognosis while the same fit patients with no donor have significantly lower survival (p<0.001) and for whom allo-HSCT could be proposed by using haploidentical donors in case of cord blood or unrelated donors absence. Interestingly, in the old population, we showed that poor prognosis patients receiving azacytidine had a significantly better survival compared to those receiving low dose Ara-C (p=0.015), with a comparable outcome to those receiving induction chemotherapy.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal