Abstract
Background: Multiple myeloma (MM) is characterized by the impaired osteogenic differentiation of mesenchymal stromal cells (MSCs). However, the underlying molecular mechanisms are unclear. Long noncoding RNAs (lncRNAs) are emerging as important regulatory molecules in tumor-suppressor and oncogenic pathways. We therefore hypothesized that lncRNAs plays an important role in regulating osteogenic differentiation of MSCs from multiple myeloma. The current studies focused on MEG3 initiated from BMP4 neighboring gene, which is involved in the regulation of osteogenic differentiation.
Methods: Expression of MEG3 and its target gene BMP4 were examined in MSCs from bone marrow of myeloma patients (n = 6) (MM-MSCs) and normal donors (n = 3) (ND-MSCs) during osteogenic differentiation. The expression of MEG3 and BMP4 were investigated using assays of quantitative real-time PCR. Correlations between MEG3 and BMP4 were assessed using Pearson's correlation coefficients. Enzyme-linked immune sorbent assay (ELISA) was performed to determine the secretion of BMP4. Luciferase assay, Chromatin immunoprecipitation (CHIP), electromobility shift assays (EMSA) and RNA immunoprecipitation (RIP) were performed to determine the mechanism of MEG3 silencing. All statistical tests were two-sided.
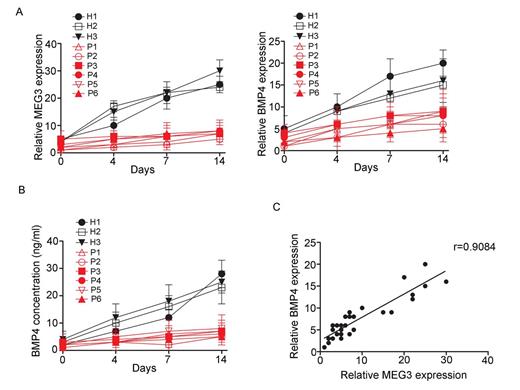
Results: Expression of MEG3 and BMP4 in ND-MSCs was increased more than 3-fold compared to that from MM-MSCs at days 4 after osteoblastic induction, and up to 4-, 5-fold of those from patients at days 7 and 14, respectively (Figure A). The synthesis and secretion of BMP4 was significantly were upregulated in ND-MSCs during osteogenic differentiation (Figure B). A positive correlation between MEG3 and BMP4 was observed in MSCs from normal donors and myeloma patients(r = 0.9084, p < 0.0001, Figure C). Intense Von Kossa staining, indicating calcification, was present in BMP4-treated MM-MSCs at day 7, day 14. But untreated MM-MSCs were negative for Von Kossa staining in osteogenic medium at day 7, day 14. In good accord with these results, several key markers of osteogenic differentiation, Runx2, Osx and OCN, were up-regulated between four and nine fold in BMP4-treated MM-MSCs at day 7 and day 14 in osteogenic medium. To evaluate the effect of MEG3 on osteogenesis, MM-MSCs with enhanced or reduced MEG3 function were developed. We observed that MEG3 knockdown significantly reduced the mRNA levels of osteogenic markers, including Runx2, Osx and OCN, while overexpression of MEG3 enhanced their expression. Additionally, MEG3 knockdown decreased BMP4 transcription. Luciferase assay revealed that MEG3 cooperated with AP-1 to increase the BMP4 transcription in MSCs. By using CHIP, EMSA and RIP assays, we showed that in MSCs MEG3 recruited the binding and action of the transcription factor AP-1 to the BMP4 promoter to increase expression of BMP4 gene. The specificity and efficiency of activation were ensured by the formation of a stable complex between MEG3 and the BMP4 promoter, direct interaction of MEG3 with AP1 and recruitment of AP1 to the promoter.
Conclusions: Taken together, our data support the hypotheses that lncRNA MEG3 promotes osteogenic differentiation of MSCs from multiple myeloma by targeting BMP4 transcription. Moreover, we showed that the mechanism of transcriptional activation of BMP4 promoter depended on MEG3 initiated from BMP4 neighboring gene and involved both the direct interaction of the AP-1 and promoter-specific activation.
Expression level of MEG3 and target gene BMP4 during osteogenic differentiation of MSCs from myeloma patients and normal donors.
Expression level of MEG3 and target gene BMP4 during osteogenic differentiation of MSCs from myeloma patients and normal donors.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal