Abstract
Impact on Survival Outcomes of Bone Marrow Plasma Cells Percentage and Morphology Evaluation by Conventional Microscopy in Multiple Myeloma after High Dose Therapy.
Background: The achievement of at least CR is a crucial step for a long-lasting response and prolonged survival after autologous stem cell transplantation (ASCT) in patients (pts) with multiple myeloma (MM). The current definition of complete remission (CR) or better in MM requires a negative serum and urine immunofixation (IF) and <5% bone marrow plasma cells (BMPCs). Additional prognostic tools are related to sFLC ratio, immunophenotypic and molecular evaluations, when possible. As BMPCs levels could differ if evaluated by BM biopsy or aspirate (the latter supposed to underestimate BMPCs count), we aim to determine a new threshold for PCs in BM aspirate and to determine whether it could be, in association with PCs morphology by standard microscopic evaluation, an easy and cheap surrogate marker for outcome, in the absence of sFLC assay and/or phenotypical-molecular analysis for MRD.
Material and Methods: 191 de novo MM pts treated between 2003-2010 in Toulouse's myeloma and BMT center with adequate clinical and biological data were retrospectively studied. Responses were evaluated at day 100 after ASCT in all pts according to IMWG criteria. BM examination comprised PCs count, BM cellularity, and the presence of PCs dysmorphy. Progression free survival (PFS) was calculated from the start of therapy until progression, death or last follow-up. Overall survival (OS) was calculated from the start of therapy until death or last follow-up. The Kaplan-Meier method was used to estimate the survival distribution.
Results: Baseline demographics and initial disease characteristics are summarized in table 1. Median follow-up is 6 years. At the completion of ASCT, 49 pts (26%) achieved CR, 89 (47%) VGPR and 41 (21%) PR; 57 pts (30%) had a negative serum IF (sIF). Overall, 151 pts relapsed and 68 died with median PFS and OS of 36 and 99 months, respectively.
At D100, median PCs count was 1% (range 0-23%): 1% (0-3%) in CR pts, 1% (0-23%) in VGPR pts, and 1.5% (0-7%) in PR pts. Only 1 pt with negative sIF had 5% BMPCs and a positive urine IF, and was assessed as VGPR. Overall, 55 negative sIF pts had 2% or less BMPCs. The number of 2% of BMPCs was found to be predictive, irrespective of response. Median PFS was 39 vs 21 months if BMPCs is > 2% (p<.001) and median OS was 99 months vs 66 (ns).
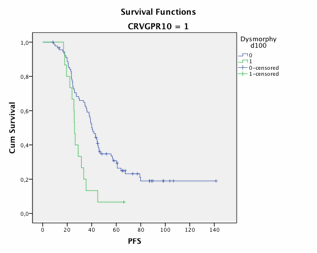
We further aimed to evaluate the impact of PCs dystrophy on survival outcomes in 176 evaluable pts. PCs dysmorphy was reported in 29 pts including 3 pts in CR, 9 VGPR and 13 PR, respectively. All except 2 pts relapsed, with a median PFS of 26 mo (vs 39, p=.002). Nineteen died with a median OS of 60 mo (vs 101, p=.003). For pts at least in VGPR, median PFS was 26 mo in case of PCs dysmorphy vs 40 mo (p=.004) and median OS was 59 mo vs not reached (p=.005). (see figures)
Conclusion: conventional microscopy of BM aspirate is a useful and rapid tool to evaluate the percentage of PCs and their morphology as a first step to assess the residual tumor mass in patients with MM after ASCT, and it constitutes a good predictor for disease progression and survival outcome. These findings have to be confirmed and the exact threshold of PCs remains to be determinate in a large prospective study.
| Characteristics . | n=191 . |
|---|---|
| Sex: M/F, n | 109/82 |
| Median age, y (range) | 57 (31–68) |
| Isotype, n (%) | |
| IgG, IgA, LC | 123 (64), 35 (18), 28 (15) |
| ISS stage, n (%) | n= 158 |
| I, II, III | 85 (54), 40 (25), 33 (21) |
| Median bone marrow plasma cells, % (range) | 23 (1-96) |
| Median b2-microglobulin, mg/L (range) | 3.1 (1.3–19.4) |
| Characteristics . | n=191 . |
|---|---|
| Sex: M/F, n | 109/82 |
| Median age, y (range) | 57 (31–68) |
| Isotype, n (%) | |
| IgG, IgA, LC | 123 (64), 35 (18), 28 (15) |
| ISS stage, n (%) | n= 158 |
| I, II, III | 85 (54), 40 (25), 33 (21) |
| Median bone marrow plasma cells, % (range) | 23 (1-96) |
| Median b2-microglobulin, mg/L (range) | 3.1 (1.3–19.4) |
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal