Abstract
Introduction
New therapies are needed for patients (pts) with chronic lymphocytic leukemia (CLL). BCL2 is an anti-apoptotic protein that is overexpressed in CLL and may be associated with resistance to available therapies; GDC-0199 is an orally available, selective BCL2 inhibitor. The combination of bendamustine (B) and rituximab (R) has demonstrated efficacy in relapsed/refractory (R/R) and previously untreated pts with CLL and is often used in these pts. Preclinical data suggest that GDC-0199 combined with BR may show synergistic activity in CLL. Moreover, clinical data from single-agent studies of GDC-0199, and in combination with rituximab in pts with CLL, support combining GDC-0199 with BR in this population. Data presented here are from an ongoing phase 1b study that is evaluating the maximum tolerated dose of GDC-0199 when given in combination with BR, as well as the safety, tolerability, and order of administration of this combination in R/R or previously untreated pts with CLL.
Methods
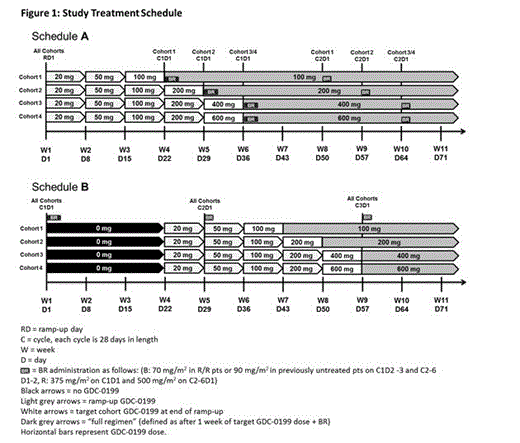
Pts with an ECOG PS ≤1, adequate marrow, hepatic, renal and coagulation function are being enrolled in dose finding cohorts ranging from 100 to 600 mg/day of GDC-0199 using a 3+3 dose escalation design. Pts are assigned to one of two dosing schedules (Figure 1) with GDC-0199 (Schedule A) or BR (Schedule B) introduced first, both incorporating a gradual dose ramp-up of GDC-0199 to reduce the risk of tumor lysis syndrome (TLS). Prophylaxis measures to mitigate TLS include IV hydration, treatment to prevent hyperuricemia and hospitalization. After completing combination therapy, R/R pts will continue single-agent GDC-0199 until disease progression. Previously untreated pts will continue single-agent GDC-0199 for up to 18 months. Dose-limiting toxicity (DLT) data are identified after all pts in a cohort have completed ³21 days of combination treatment at the target dose of GDC-0199 and focus on treatment emergent TLS and cytopenias.
Results
As of May 2014, 6 pts with R/R CLL have been enrolled in the dose finding stage of the study with a median time on study of 67 (range 43-113) days. All 6 pts were enrolled on Schedule A; 3 pts were enrolled in the 100 mg GDC-0199 cohort and 3 pts were enrolled in the 200 mg cohort. Baseline characteristics of the 6 pts are as follows: median age 67 (range 52-71) years, 3 male pts, median of 2 prior CLL therapies (range 1-3, including 4 pts with previous exposure to fludarabine, cyclophosphamide and rituximab [FCR] and 1 with previous exposure to FCR and B), beta-2 microglobulin ≥3.5 mg/L in 2 pts, and unmutated IGHV in 5 pts. Prior to starting therapy, the median lymphocyte count was 40.85 x 109 cells/L (range 2.11-169 x 109 cells/L), hemoglobin level was 101.5 g/L (range 99-134 g/L), neutrophil count was 2.54 cells/μL (range 1.81-3.8 cells/μL), and platelet count was 83.5 x 109/L (range 49-250 x 109/L). Pts were assigned to 1 of 3 TLS risk groups based on screening ALC and tumor bulk: low risk 1 pt, medium risk 4 pts, and high risk 1 pt. Cytogenetic data are available for 3 pts: 1 pt had del17p, 2 pts had del 11q, and 3 pts had del 13q.
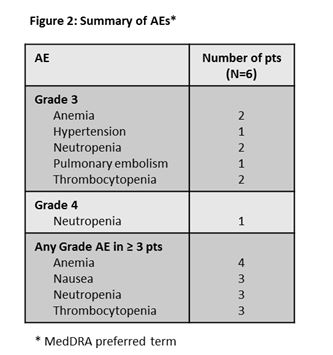
No DLTs were observed. All 6 pts have experienced adverse events (AEs, Figure 2). The most common AE was anemia with most Grade ³3 AEs being hematological toxicities (Figure 2). One serious AE of bronchitis was observed in a pt enrolled in the 100 mg GDC-0199 cohort and resolved after treatment. Two pts in the 100 mg GDC-0199 cohort discontinued BR after the 21-day DLT window secondary to treatment emergent cytopenia: 1 with neutropenia after completing 1 cycle of BR (previous treatment FCR without baseline cytopenia) and the second with thrombocytopenia after completing 3 cycles of BR (previous treatment FCR and chlorambucil with thrombocytopenia at screening). Both pts remain on single-agent GDC-0199. No TLS events (laboratory or clinical) were observed and no deaths reported.
Conclusion
This is the first study to evaluate the combination of GDC-0199 and BR in pts with CLL. Despite 5 pts being identified as medium or high risk for TLS, no pts developed TLS as a result of the ramp-up dosing of GDC-199 and prophylactic measures. The most frequent AEs were hematological toxicities and generally manageable; however, 2 pts had to discontinue BR (after 1 and 3 cycles) but were able to remain on GDC-0199. Dose escalation in R/R pts is continuing in order to evaluate the optimal dose/schedule with a plan to enroll previously untreated pts in the near future.
Boyd:Genentech: Research Funding; US Oncology/McKesson: Research Funding; Celgene: Consultancy. Morschhauser:Genentech: Travel grant Other; Gilead, Mundipharma, Bayer, Spectrum, Celgene: Honoraria. Wendtner:AbbVie, Genentech, Hoffman-La Roche, Mundipharma: Consultancy, Research Funding. Lymp:Genentech: Employment. Hilger:Genentech: Employment. Vosganian:Genentech: Employment. Huang:Genentech: Employment. Stilgenbauer:Genentech, Hoffman La Roche: Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal