Abstract
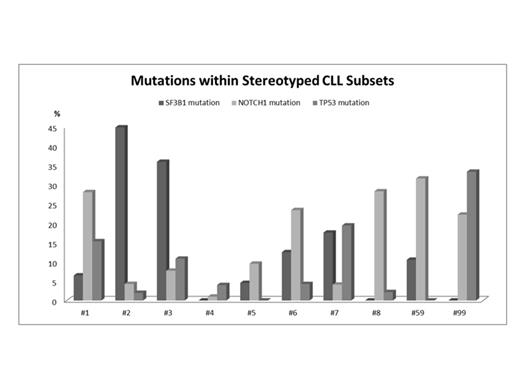
Preliminary observations from essentially small patient series indicate that certain recurrent gene mutations may be enriched in subsets of chronic lymphocytic leukemia (CLL) with stereotyped B-cell receptors (BcR). On these grounds, it could be argued that differential modes of immune signaling, in the context of subset-biased antigen-immunoglobulin (IG) interactions, may be associated with the acquisition and/or selection of certain genomic aberrations within various stereotyped CLL subsets. With this in mind, we here sought to explore the genetic background of 10 major stereotyped subsets which collectively account for ~11% of all CLL and represent both IGHV unmutated (U-CLL) and/or mutated (M-CLL) cases. We focused on recurrent mutations within the NOTCH1 (entire exon 34 or targeted analysis for del7544-45), TP53 (exons 4-9), SF3B1 (exons 14-16), BIRC3 (exons 6-9) and MYD88 (exon 5) genes. Overall, 647 cases were analyzed, belonging to the following major subsets: (i) U-CLL: #1 (the largest within U-CLL, clinically aggressive), n=139; #3, n=39; #5, n=22; #6, n=48; #7, n=74; #8, n=46; #59, n=19 and #99, n=18; (ii) M-CLL: #4 (the largest within M-CLL, particularly indolent), n=78; and, (iii) subset #2 (the largest overall, variable mutational status and clinically aggressive), n=164. All cases were devoid of MYD88 mutations, which was not surprising given that our cohort was predominantly composed of U-CLL. Mutations within the BIRC3 gene were either absent (#2, #4, #6 and #59) or rare (#1, #3, #5, #7, #8 and #99; frequency 1.5%-7%) with no clear bias to any subset. BIRC3-mutant cases frequently co-existed with either del(11q) or trisomy 12. NOTCH1 mutations were more frequent in subsets #1, #6, #8, #59 and #99 (frequency, 22%-32%), sharply contrasting subsets #2 or #3 (4% and 7%, respectively) (p<0.0001). Of note, although NOTCH1 mutations tended to coincide with trisomy 12 in certain subsets e.g. #1 and #8, their co-occurrence differed significantly with only 33% of NOTCH1mut subset #1 cases carrying trisomy 12 compared to 75% of NOTCH1mut subset #8 cases (p=0.036). Moving to SF3B1, we noted that subsets harboring NOTCH1 mutations were either absent for or carried few SF3B1 mutations, while the inverse was also true i.e. very high frequency of SF3B1 mutations in subsets #2 and #3, 45% and 36%, respectively. Almost 80% of mutations observed in subset #2 were localized to two codons (p.K700E: n=44/76, 58%: p.G742D: n=15/76, 20%) within the HEAT domain of the SF3B1 protein; p.K700E accounted for only 29% (4/14) of all SF3B1 mutations detected in subset #3 while p.G742D was absent (p=0.043 and p=0.068 respectively). Thus, although the functional relevance of these mutations is currently unknown, their high frequency and striking bias to subset #2 bodes strongly for their critical role in the pathobiology of subset #2. Finally, TP53 mutations were: (i) enriched in subsets #3 (11%) and #7 (19%) and, in contrast, absent or rare in subsets #5 (0%) and #6 (4%), despite all utilizing the IGHV1-69 gene (p=0.02); (ii) enriched in subset #1 (15%) and subset #99 (33%), a less populated subset that is highly similar to subset #1; and, (iii) very rare in subsets #2 and #8 (2% in both), the latter known to display the highest risk for Richter's transformation among all CLL. In conclusion, we confirm and significantly extend recent observations indicating that different CLL stereotyped subsets display distinct genetic makeup. These findings imply that distinctive modes of microenvironmental interactions, mediated by certain stereotyped BcRs, may be associated with selection or occurrence of particular genetic aberrations, with the combined effect determining both clonal and clinical evolution, and ultimately disease outcome.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal