Abstract
Introduction:
Hypocellular myelodysplastic syndrome (hMDS) is characterized by decreased marrow cellularity, and is often difficult to distinguish from aplastic anemia (AA) based on standard morphological criteria. It represents around 10-15% of patients diagnosed with MDS, but is not currently considered a separate entity by WHO. Hypocellularity is defined as bone marrow cellularity of less than 30% in patients younger than 70 years or less than 20% in those older than 70 years.
Patients and Methods:
We retrospectively evaluated the demographic, clinical features, bone marrow aspirate/trephine, treatment characteristics and outcomes of 100 patients with hMDS. The bone marrow aspirates and trephines were also analysed for dysplasia, blast percentage, cellularity, reticulin, p53 by Immunohistochemistry (IHC), CD34 and CD117. An MiSeq based targeted gene panel comprising of 24 frequently mutated MDS genes was used in a subset of patients.
Results:
The median age was 51 years (18-87), with only a third of patients >60 years. WHO subtypes RA (n=2), MDS 5q- (n=1), RCMD (n=95) and RAEB (n=2), IPSS risk groups low risk (n=20), Int1 (n=61), int2 (n=11) and high risk (n=2). 23% had evolved from previous AA.
For the hMDS group IPSS cytogenetic categories comprised good risk 67% (n=62), intermediate 14% (n=13), including 6 cases of trisomy 8, and poor 18% (n=17), with 7 cases of monosomy 7. Of the normal cytogenetics, 15/62 (24%) had positive (moderate to strong) p53 by IHC, 30/62 (48%) with CD117 positivity (1-20%), 48/62 (77%) have fibrosis grade 1-2. Only 2/62 with normal cytogenetics had normal p53, CD34, CD117 and reticulin and these have evidence of dysplasia on morphology. The presence of cytogenetic abnormalities and other features such as p53, CD117 and fibrosis reflect a distinct population differing from the hypocellular marrow of aplastic anemia.
A higher incidence (18%) of autoimmune disorders was seen, including ITP (n=2), thyroid dysfunction (n=3), alopecia (n=2), inflammatory bowel disease (n=4), coeliac (n=2), SLE/SjogrenÕs (n=2), others (n=6).
Forty percent (n=32) had a PNH clone, all except 2 were subclinical. Progression of disease to AML (n=3), upstaging of disease (RCMD to RAEB, n=6) or cytogenetic evolution (n=3) was seen in 15% of hMDS, including 3 cases with both increase in blasts and karyotypic evolution.
A subset (n=33) of hMDS were evaluated for recurrently mutated genes in MDS; 7/33 (21%) of patients harbored somatic mutations in ASXL1 (n=4), DNMT3A (n=2) and BCOR (n=2). All except one patient with somatic mutation had a prior history of aplastic anaemia.
As the predominant group was hypocellular RCMD (95%), we compared the clinical features, treatment and prognosis with cellular RCMD cohort during the same time period.
Median ages of hMDS and non-hypocellular MDS (nhMDS) were 51 and 60.3 years respectively. Patients with hMDS presented with more significant thrombocytopenia (median platelet count at presentation 43 vs 93), neutropenia (median ANC at presentation 1.13 vs 1.3), anaemia (median Hb 94g/L vs 104g/L), transfusion dependency, and more intermediate-2/high-risk disease than the nhMDS group (p=0.0257). Among hMDS patients, 30% (28/95) had chromosomal abnormalities, an incidence similar to that of nhMDS, 25% (23/94).
Treatments received by hMDS cohort were: single agent Cyclosporin (n=27), anti-thymocyte globulin (n=7), erythropoietin and GCSF (n=13), 5-azacytidine (n=6), intensive chemotherapy (n=4), HSCT (n=26), including 9 patients who underwent upfront HSCT. As expected the use of IST was infrequent (8%) in the nhMDS cohort compared to 35% in the hMDS (p<0.03).
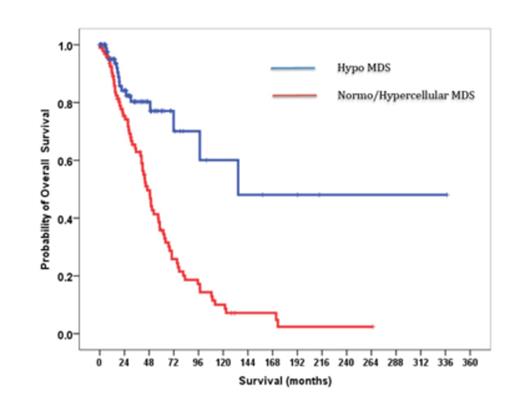
The rate of progression to acute myeloid leukaemia was lower in hMDS compared to nhMDS group (3% vs 13%, p<0.02). The median overall survival was longer for patients with hMDS compared with the nhMDS (11.1 years vs 4.3 years p<0.001).
Conclusion:
This large study of hMDS identifies a specific subgroup of MDS with lower median age, severe cytopenia, high risk disease and a good prognosis. The increased incidence of trisomy 8 and autoimmune disorders is indicative of immunological dysfunction in this group of patients. The presence of specific genomic abnormalities (ASXL1, DNMT3A, BCOR), especially in patients with prior history of aplastic anaemia needs further comprehensive evaluation in a prospective study.
Hypocellular MDS vs Normo/hypercellular MDS (median OS 11.1 vs 4.3 years, p<0.001)
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal