Abstract
Introduction
Young adults with Essential Thrombocythemia (ET) or early Primary Myelofibrosis (early-PMF) are a category of patients projected to a prolonged survival but also to an extended utilization of medical resources. Mutations, including those in the calreticulin (CALR) gene, have been reported to affect main clinical features and outcome in large cohorts of patients with Ph-negative MPNs. However, no data are available on mutational status and long-term outcome in young MPN patients.
Methods
A clinic-pathologic database of ET patients followed in 5 Italian Hematology Centers was created. A total of 217 WHO-diagnosed ET or early-PMF patients ≤ 40 years at diagnosis was retrieved from the general database of 2635 patients. All bone marrow biopsies were reviewed at local institution. Baseline clinical/molecular characteristics and outcome measures (thrombosis, hemorrhages, secondary MF and AL, second neoplasia, death, overall and event-free survival) were evaluated. JAK2V617F allele-burden was assessed in granulocyte DNA by using ipsogen JAK2 MutaQuant Kit (qPCR). CALR mutations were identified by next generation sequencing (NGS) approach on GS Junior (Roche-454 platform); MPL mutations were evaluated by using ipsogen MPLW515L/K MutaScreen Kit.
Results
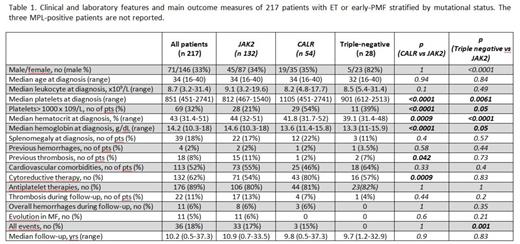
Overall, 197 WHO-defined ET and 20 early-PMF (age range: 16-40, median 34) were included in the study. Mutational frequencies were 61% for JAK2, 25% for CALR, 1% for MPL and 13% for triple negative. Baseline clinical characteristics and use of antiplatelet/cytoreductive therapies were comparable in ET and early-PMF, although frequency of triple negative was higher in the early-PMF cohort.
Compared to the JAK2 positive population, both CALR and triple-negative patients showed higher platelet count and lower hemoglobin and hematocrit levels (Table 1).
Median follow-up was 10.2 years (range: 0.5-37.5). During follow-up, 19 (9,6%) ET and 3 (15%) early-PMF patients experienced a total of 31 thrombotic (arterial: 38%) and 12 hemorrhagic events, with an incidence rate of 0.91% and 0.39% patients/yr, respectively. The cumulative incidence of thrombosis was 0,14% and 0,24% at 15 and at 20 years, respectively.
Overall, 10 patients (4,6%) and 1 (0,4%) patients evolved to MF and AL, respectively; 10 developed a second neoplasia. The cumulative incidence of disease progression into MF/AL was 0,03% and 0,13% at 15 and at 20 years, respectively. At last contact, 6 (2,7%) patients had died, at a median age of 61 years (20-71), for an overall survival of 98% at 15 years. Causes of death were related to the myeloproliferative neoplasm (disease evolution or thrombotic complications) in all patients but one. Event-free survival was similar in the ET/early-PMF cohorts considering both every event separately and all together.
In univariate analysis, male sex (p=0.003), previous thrombosis (p=0.001), splenomegaly (p=0.037), JAK2V617F (p=0.019) were associated with increased thrombotic risk; in multivariate Cox analysis, only previous thrombosis and male sex remained significant (p=0.012). Baseline splenomegaly was the only predictive factor for subsequent hemorrhages (p=0.017). Abnormal karyotype was associated with secondary MF (p=0.013) and also with second neoplasia (p<0.001). Together with JAK2V617F positivity and leukocytosis >11x109/L, abnormal karyotype was also associated with worse survival in univariate analysis. However, in multivariate analysis only JAK2V617F mutation remained as negative predictor of survival (p=0.019). Also, multivariable analysis confirmed JAK2 mutation and splenomegaly as independent risk factors for cumulative events.
Conclusions
With the limitations due to the low number of early-PMF, the outcome of young adults with early-PMF and true ET seemed to be comparable. The correlation of abnormal karyotype with MF transformation and second neoplasia suggests the need for an accurate cytogenetic analysis at diagnosis. Mutational status did influence disease phenotype, in terms of baseline characteristics and prognosis. Indeed, JAK2 mutational status confirmed a negative prognostic role for thrombosis and survival, while event-free survival was significantly better in triple-negative patients. Notably, causes of death were mostly related to the hematological malignancy, pointing out the substantial impact that this generally indolent disease may acquire in young adults.
Latagliata:Novartis: Consultancy; Bristol Myers-Squibb: Consultancy; Celgene: Consultancy; Shire: Consultancy. Cavo:Janssen: Consultancy, Speakers Bureau; Celgene: Consultancy, Speakers Bureau; BMS: Consultancy, Honoraria; Millenium: Consultancy, Honoraria; Onyx: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal