Abstract
Introduction: The introduction of continuous tyrosine kinase inhibitor (TKI) treatment has dramatically improved progression-free survival for chronic phase chronic myeloid leukaemia (CML) patients. This success, however, has put the issue of long-term drug toxicity and safety into focus. Recent data from clinical studies have indicated an increased risk of cardiovascular events (CVE), including peripheral arterial occlusive disease, in CML patients receiving treatment with the TKIs nilotinib or ponatinib, as compared to imatinib (Giles et al, Leukemia 2013; Kim et al, Leukemia 2013; Cortes et al, New England Journal of Medicine 2013; FDA communication 2013).
This study used data retrieved from Swedish population-based registries to estimate the frequency of CVE in CML patients, particularly those treated with imatinib and the 2nd generation TKIs nilotinib and dasatinib.
Methods: We identified all incident cases between 2002 and 2012 in the Nationwide Swedish CML register. All patients who were in blast crisis or accelerated phase at time of diagnosis were excluded. All patients were followed untill death, emigration or 31st December 2012. For all CML patients a comparison cohort was established, matched to be of the same age and sex as the CML cohort, with 5 control subjects per CML patient. By means of record linkage with the nationwide Swedish patient register both cohorts were followed for the occurrence of adverse cardiovascular outcomes. Two sets of relative risks (expressed as incidence rate ratios; IRRs) of cardiovascular and venous thromboembolic disease were computed. In a first step CML patients were compared to the control population. In a second step, restricted to CML patients ever treated with TKIs, CML patients on different TKI treatments were compared. Patients could be treated with several TKIs during their follow-up, and events would only be attributable to the TKI used during the time period. Both analyses were adjusted for age, sex and calendar period. The second analysis was also adjusted for Sokal risk score.
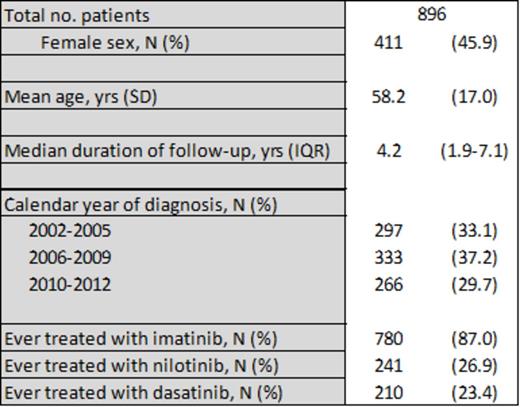
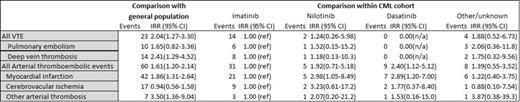
Results: A total of 896 CML patients were included and followed during a median of 4.2 years (Table I). The main outcome data are presented in Table II. A total of 23 venous thrombotic events (VTE) and 60 arterial thrombotic events were detected in the CML patient cohort during follow-up. Compared with the general population, this corresponded to significantly increased risks. In particular, deep venous thrombosis and “other arterial thromboses” were more common among CML patients (IRR 2.41 95% CI 1.29-4.52 and IRR 3.50 95% CI 1.36-9.04, respectively). Assessing risks associated with particular TKIs, we noted that treatment with any of the 2nd generation TKIs nilotinib or dasatinib, as compared to imatinib, was associated with a significantly increased occurrence of myocardial infarction (IRR 2.98 95% CI 1.05-8.49 and IRR 2.89 95% CI 1.20-7.00, respectively). Notably, there were no differences in the occurrence of CVE between the different patient groups before CML diagnosis.
Conclusion: These data, derived from a large population-based Swedish cohort, provide evidence of an increased risk of both venous and arterial thrombotic events among CML patients and that patients on 2nd generation TKIs, as compared to imatinib, may be at increased risk of myocardial infarction. Further analyses will assess whether these differences may reflect patient selection and characterstics, rather than drug-related factors. Meanwhile, risk factors for CVE should be observed and considered in the TKI treatment of CML.
* Footnote: the number of events may not add up because of occurrence of more than one type of vascular event in one subject. The number of events in the analysis within the CML cohort is lower than in the comparison with the general population because of exclusion of patients who were never treated with TKIs in the former analysis.
Björkholm:Novartis: Research Funding; Shire: Research Funding; Merck: Research Funding; Amgen: Honoraria, Research Funding; Pfizer: Research Funding; Bristol-Myers Squibb: Honoraria; Celgene: Honoraria; Akinon: Honoraria; Nordic Nanovector: Honoraria. Själander:Novartis: Honoraria. Richter:Ariad: Honoraria; Bristol-Myers Squibb: Honoraria; Novartis: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal