Abstract
DT2219 is a recombinant fusion protein composed of catalytic and translocation domains of diphtheria toxin (DT) fused with bispecific single chain variable fragments (sFv) of antibodies targeting human CD19 and CD22 cell surface receptors. Preclinical testing determined that the combination of two different sFvs and a toxin on the same single-chain molecule resulted in efficient toxin delivery and greater anticancer activity compared to monomeric immunotoxins sFv alone or anti-CD19 and anti-CD22 parental monoclonal antibodies. Murine studies demonstrated clinical efficacy and enhanced therapeutic effect with repetitive dosing (Vallera D, Clin Cancer Res 2009).
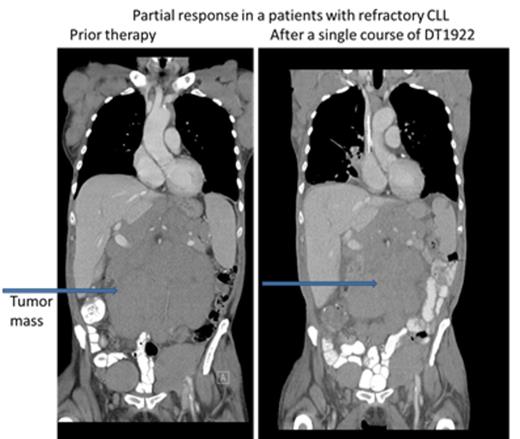
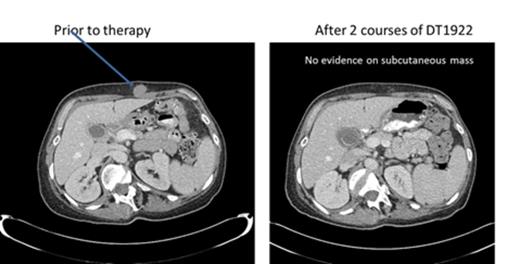
We conducted a phase 1 study to assess safety, to determine the maximum tolerated dose (MTD) and to evaluate preliminary efficacy in patients with chemotherapy refractory B cell leukemia or lymphoma expressing CD19 and/or CD22. DT2219 was administered in a single course intravenously over 4 hours (1-2 hours for subsequent doses) every other day for 4 total doses. The starting dose was 0.5 µg/kg/dose (2.0 ug/kg/course) (1/1000th of the MTD in rabbits). The dose was escalated by accelerated design until dose limited toxicity (DLT) was observed. Twenty-two patients were enrolled, with a median age of 55 years (range, 34-78). Five patients had pre-B acute lymphoblastic leukemia (4 refractory, 1 with extra-medullary disease, 1 had chronic lymphocytic leukemia (CLL) and 7 had non-Hodgkin lymphoma). All were chemo-refractory with a median of 4 prior therapies. All patients had biopsy-confirmed tumor expression of CD19 and/or CD22. The first 9 patients were treated at doses ranging from 0.5 ug/kg/dose to 20 ug/kg/dose. No drug was detectable in those patients and they experienced no DLT or clinical responses. DT2219 was detectable in the serum at doses ≥40 µg/kg/dose (N=13). Dose levels were 40 µg/kg/day N=5; 60 µg/kg/day N=5; 80 µg/kg/day N=3. The area under the curve (AUC ) range was 1,104 -1,346 and the half-life ranged from 54-84 minutes. After a single course of DT2219 we observed partial remission in a 77 year old patient with rituximab/chemotherapy-refractory CLL (dose level 40 µg/kg). The 40% reduction in the abdominal tumor mass is shown in Figure 1. This patient did not experience any DLT. A second partial remission was observed in 53 year old patient with relapsed marginal zone lymphoma (dose level 60 µg/kg) who experienced a DLT of capillary leak syndrome. After regulatory approval, that patient received a second treatment course 8 weeks later at a reduced dose of 40 µg/kg which resulted in a complete remission (Figure 2). Both patients are alive and in remission at 6 and 4 months, respectively, after therapy. Adverse events were observed in all patients who received ≥40 µg/kg. The most common was grade 1-2 capillary leak syndrome, grade 1-2 hematologic toxicity, elevated liver function tests and fatigue. Two patients experienced DLTs: at the 40 µg/kg dose (grade 3 lower extremity weakness) and 60 µg/kg dose ( grade 4 neutropenia and grade 3 capillary leak). All adverse reactions resolved completely within one week. Immunogenicity and formation of neutralizing antibodies is a major barrier in development of DT-based immunotoxins. Remarkably, none of the 8 patients with B-cell lymphoma/CLL who had received rituximab within weeks preceding therapy with DT2219 had detectable neutralizing antibody. These patients had no detectable B cells at the time of study drug administration. This suggests that rituximab pre-treatment may prevent the development of neutralizing antibodies and allow for repetitive dosing. In conclusion, we have demonstrated promising clinical efficacy of the novel immunotoxin DT2219 in refractory B cell lymphoid malignancies. We determined that the biologically active dose of DT2219 is between 40-80 µg/kg and that repetitive dosing is feasible in patients pre-treated with rituximab. Phase 2 trial is in development.
CT images of 77 year old patient with refractory CLL after a single course of DT1922 at dose level 40 µg/kg.
CT images of 77 year old patient with refractory CLL after a single course of DT1922 at dose level 40 µg/kg.
53 year old female with CD22+CD19+ relapse marginal zone lymphoma received 2 courses of DT1922 ( 60 µg/kg and 40 µg/kg) and attained a complete resolution of subcutaneous tumor mass.
53 year old female with CD22+CD19+ relapse marginal zone lymphoma received 2 courses of DT1922 ( 60 µg/kg and 40 µg/kg) and attained a complete resolution of subcutaneous tumor mass.
Vallera:The Lion Fund, William Lawrence and Blanche Hughes Foundation : Other, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal