Abstract
Background: Systemic anaplastic large cell lymphoma (sALCL) is a CD30-positive aggressive subtype of mature T-cell lymphoma. Approximately 50% of patients (pts) with sALCL develop recurrent disease after frontline treatment (Savage, 2008). Outcomes have historically been poor for pts with relapsed T-cell lymphomas, including sALCL, with a median overall survival (OS) and progression-free survival (PFS) of 5.5 months (mos) and 3.1 mos, respectively (Mak, 2013). A phase 2 study evaluated the efficacy and safety of brentuximab vedotin, a CD30-directed antibody-drug conjugate, in pts with relapsed or refractory sALCL (ClinicalTrials.gov #NCT00866047). Four-year follow-up data from this ongoing trial are presented.
Methods: Pts received 1.8 mg/kg brentuximab vedotin every 3 weeks as a 30-minute outpatient IV infusion for up to 16 cycles. Response was assessed according to the Revised Response Criteria for Malignant Lymphoma (Cheson 2007). Assessments of response and durability of response per an independent review facility (IRF) have been previously reported. Following a protocol amendment that removed the requirement for routine CT scanning during the follow-up period, response is now being assessed per the investigator. Survival and disease status are being assessed every 3 mos for 2 years, every 6 mos during years 3 to 5, and annually thereafter. CT scans are required if progression is suspected clinically.
Results: The enrolled population of 58 pts was heavily pretreated with poor prognosis. As previously reported, 72% of patients had ALK-negative disease, 62% had primary refractory disease (defined as no complete remission [CR] or relapse within 3 months of frontline therapy), and 26% had failed a prior autologous stem cell transplant (SCT). Pts had received a median of 2 prior systemic chemotherapy regimens (range, 1 to 6). Per investigator, the objective response rate (ORR) with brentuximab vedotin was 83% (48 pts) and the CR rate was 62% (36 pts), which were similar to the previously reported ORR (86%) and CR (59%) rates per IRF.
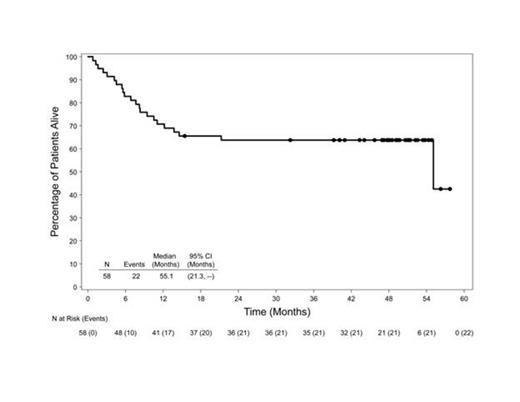
At the time of this analysis (data cut June 2014), all pts had discontinued treatment and the median observation time from first dose was 46.3 mos (range, 0.8 to 57.7). Sixty-two percent (36 of 58) of pts were alive at last follow-up and the estimated 4-year survival rate by Kaplan-Meier analysis was 64% (95% CI: 51%, 76%). Median OS by best clinical response was CR (n=36): median not reached; partial remission (n=12): 11.6 mos; stable disease (n=4): 6.9 mos; and progressive disease (n=2): 4.2 mos. Median PFS was 20.0 mos (95% CI: 9.4, – [range, 0.8 to 54.9+]) for all pts and was not reached in pts with CR. Median PFS for pts with ALK-positive (25.5 mos) and ALK-negative (20.0 mos) disease were similar. Median PFS for pts with PET-negative disease at Cycle 4 (n=28) was not reached, whereas median PFS for pts with PET-positive disease at Cycle 4 (n=20) was 6.7 mos. After discontinuing treatment, 18 pts received a hematopoietic SCT (9 allogeneic, 9 autologous). The median PFS for the pts who achieved a CR and did not receive a post-treatment SCT (n=21) was 37.7 mos (95% CI: 14.1, - [range, 2.8 to 51.1+]) and the median PFS was not reached for the pts who achieved a CR and received a subsequent SCT (n=15) (95% CI: 9.5, - [range, 8.0 to 54.4+]). Of the 36 pts who achieved CR per the investigator, 17 (47%) remain in follow-up free of progression: 10 pts received a consolidative SCT following treatment with brentuximab vedotin and 7 pts received no further therapy after completing brentuximab vedotin treatment.
As previously reported, adverse events (AEs) in ≥20% of pts were peripheral sensory neuropathy, nausea, fatigue, pyrexia, diarrhea, rash, constipation, and neutropenia. AEs ≥ Grade 3 that occurred in ≥5% of pts were neutropenia, thrombocytopenia, peripheral sensory neuropathy, anemia, recurrent ALCL, and fatigue.
Conclusions: After a median observation time of approximately 4 years from first dose of brentuximab vedotin, the 4-year survival rate was 64%. Forty-seven percent of patients with CR remain in follow-up with no evidence of progression, suggesting that brentuximab vedotin treatment may be curative for some patients. A randomized phase 3 study is being conducted to evaluate brentuximab vedotin in combination with cyclophosphamide, doxorubicin, and prednisone for frontline treatment of CD30-positive mature T-cell lymphomas, including sALCL (ClinicalTrials.gov #NCT01777152).
Pro:Seattle Genetics, Inc.: Consultancy, Research Funding, Travel expenses Other. Advani:Takeda Pharmaceuticals International Co.: Research Funding; Celgene: Research Funding; Pharmacyclics: Research Funding; Janssen Pharmaceuticals: Research Funding; Genentech: Research Funding; Seattle Genetics, Inc.: Other, Research Funding. Brice:Seattle Genetics, Inc.: Research Funding; Takeda Pharmaceuticals International Co.: Honoraria, Research Funding; Roche: Honoraria. Bartlett:Genentech: Research Funding; ImaginAb: Research Funding; Celgene: Research Funding; MedImmune: Research Funding; Novartis: Research Funding; Pharmacyclics: Research Funding; Pfizer: Research Funding; Takeda Pharmaceuticals International Co.: Research Funding; Seattle Genetics, Inc.: Other, Research Funding; Janssen: Research Funding; AstraZeneca: Research Funding. Rosenblatt:Seattle Genetics, Inc.: Research Funding; University of Miami: Employment. Illidge:Seattle Genetics, Inc.: Consultancy, Research Funding; Takeda Pharmaceuticals International Co.: Consultancy, Honoraria. Matous:Seattle Genetics, Inc.: Research Funding, Speakers Bureau; Celgene: Consultancy, Speakers Bureau; Onyx: Speakers Bureau; Takeda Pharmaceuticals International Co.: Speakers Bureau. Ramchandern:Seattle Genetics, Inc.: Research Funding, Speakers Bureau. Fanale:Seattle Genetics, Inc.: Consultancy, Honoraria, Other, Research Funding. Connors:Seattle Genetics, Inc.: Research Funding; Roche: Research Funding. Wang:Seattle Genetics, Inc.: Employment, Equity Ownership. Huebner:Takeda Pharmaceuticals International Co.: Employment, Equity Ownership. Kennedy:Seattle Genetics, Inc.: Employment, Equity Ownership. Shustov:Seattle Genetics, Inc.: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal