Abstract
Background: A number of second-generation monoclonal antibodies (mAbs) that target the antigens CD20 and CD19 have been evaluated across a range of non-Hodgkin's lymphoma (NHL) subtypes combined with chemotherapy, as a single agent, or as maintenance therapy. Although these agents are usually well tolerated and have demonstrated clinical activity in patients (pts) with NHL, there is still a high unmet medical need for pts with refractory or relapsed NHL. MOR00208 is an Fc-engineered humanized mAb that targets the antigen CD19 and possesses significantly enhanced antibody-dependent cell-mediated cytotoxicity, a key mechanism for tumor cell killing.
Methods: This non-randomized, phase IIa, open-label, multicenter study (NCT01685008) was designed to assess the efficacy and safety of single-agent MOR00208 in pts ≥18 years with relapsed or refractory NHL previously treated with rituximab, who were not candidates for high-dose therapy with stem cell support. Pts with diffuse large B-cell lymphoma (DLBCL), follicular lymphoma (FL), mantle cell lymphoma (MCL), and other indolent NHL (iNHL), who had received at least one prior rituximab-containing therapy, were eligible. Additional eligibility criteria included European Cooperative Oncology Group Performance Status (ECOG PS) 0-2, measurable disease ≥1.5 cm, adequate bone marrow function, renal and liver function test. Pts were treated with MOR00208 12 mg/kg IV weekly for a total of 8 doses. Those with at least stable disease according to International Response Criteria (Cheson, JCO 2007), were to continue MOR00208 treatment for an additional 4 weeks. After completion of these 12 weekly doses of treatment, responding pts (complete or partial remission [CR or PR]) received maintenance MOR00208 every 2 weeks until progression. The study utilized a 2-stage design. In stage 1, ≥10 pts were to be enrolled in each of the 4 NHL disease cohorts. Cohorts with ≥2 responses (CR or PR) in stage 1 were expanded by an additional 20 patients in stage 2. Disease cohorts with <2 responses in stage 1 were stopped for futility. Enrollment of a minimum of 40 and a maximum of 120 pts was planned.
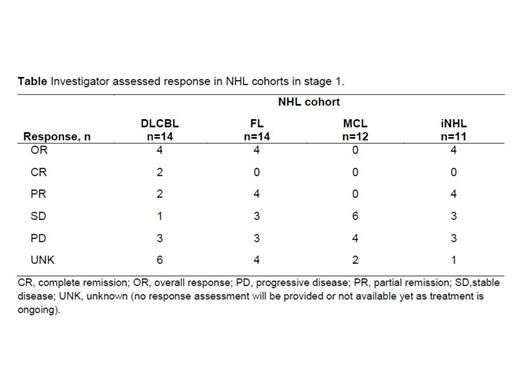
Results: Herein we report the efficacy results for the 51 pts enrolled in stage 1 and the safety for 85 pts enrolled to date in stages 1 and 2 of this trial. The median age for all 51 stage 1 pts was 70 (35–90) years, 46 had stage III-IV disease, and the median number of prior therapies was 3 (2-4). Treatment cohorts consisted of DLBCL (n=14), FL (n=14), MCL (n=12) and iNHL (n=11). The investigator assessed overall response rate in stage 1 over all NHL subtypes was 24% (12/51), with responses observed in the DLBCL, FL, and iNHL cohorts (Table). As of February 2014, 62/85 (72.9%) stage 1 and stage 2 pts had developed treatment emergent adverse events (TEAEs). The most frequently reported TEAEs of any grade were thrombocytopenia (11.8%), anemia and headache (10.6% each), and neutropenia (9.4%). Infusion-related toxicities were all grade 1-2 (except for one case of dyspnea of grade 4). There have been no treatment-related deaths. Most of the TEAEs occurred in pts with DLBCL (30/36 [83.3%]). In pts with DLBCL, the most frequently reported grade ≥3 TEAEs were neutropenia (13.9%), thrombocytopenia and anemia (8.3% each).
Conclusion: The Fc-engineered humanized anti-CD19 antibody, M0R00208, is well tolerated without significant infusional toxicity and demonstrates preliminary efficacy in pts with relapsed/refractory DLBCL, FL, and iNHL. Accrual to stage 2 for pts with DLBCL and FL is ongoing.
Blum:MorphoSys: Research Funding. Maddocks:MorphoSys: Research Funding; Pharmacyclics: Research Funding; Seattle Genetics: Research Funding; Pharmacyclics: Other. Nagy:AMGEN: Consultancy. Zinzani:MorphoSys: Membership on an entity's Board of Directors or advisory committees. Robak:MorphoSys AG: Research Funding. Korolkiewicz:MorphoSys AG: Employment. Winderlich:MorphoSys: Employment, Patents & Royalties. Jurczak:Celgene: Research Funding; Eisai: Research Funding; Gilead: Research Funding; Jansen: Research Funding; Pharmacyclics: Research Funding; Pfizer: Research Funding; Roche: Research Funding; Sandoz-Novartis: Research Funding; Spectrum: Research Funding; Takeda: Research Funding; Teva: Research Funding; Mundipgharma: Consultancy; Spectrum: Consultancy; Teva: Consultancy; Takeda: Consultancy; Roche: Consultancy; Sanoz-Novartis: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal