Abstract
Introduction: The role of brentuximab vedotin (BV) in Hodgkin Lymphoma (HL) is expanding, but factors predicting progression-free survival (PFS) after BV therapy are poorly defined. Age, tumor bulk, presence of extranodal disease, neutrophil:lymphocyte ratio (ANC/ALC), and lymphocyte:monocyte ratio (ALC/AMC) predict outcome in HL patients (pts) treated with chemotherapy, but their impact on PFS after BV has not been well-studied. Also, among pts with relapsed/refractory HL (rel/ref HL) who progress after BV, efficacy of additional chemotherapy is undefined. To inform patient selection and future clinical trial design with BV, we undertook a retrospective study to identify factors predicting PFS with BV therapy in rel/ref HL, and explore chemotherapy efficacy as salvage after BV failure.
Methods: Pts receiving BV since 2009 were identified through pharmacy and research records and studied with IRB approval. Those with rel/ref HL receiving BV before or after transplant without intervening therapy were excluded. Age ≥40 at time, sex, pre-BV PET findings (SUV max, extranodal [EN] involvement, bulk > 5cm), prior therapy (# lines of therapy> median; prior transplant, platinum-containing, radiotherapy), and lab findings (AMC/ALC³4.3, ALC/AMC ratio³1) at time of start of BV were examined for an impact on PFS and OS via log-rank testing of Kaplan-meier projections(JMP 11.0 software). PFS was defined as time from first BV dose to radiographic or clinical progression, initiation of post-BV salvage, or death from any cause. OS was measured from date of first BV dose to death from any cause. Efficacy of salvage therapy for those failing BV was recorded.
Results: Of 90 patient receiving BV, 43 met above criteria. Median age was 34 yrs (range 17-80), median # of pre-BV therapies was 3 (range 1-7). 31 (73%) had failed autologous transplant, 10 (23%) had undergone allogeneic transplant, and 20 (46%) received radiotherapy prior to BV. Pre-BV PET staging data was available in 26 pts; post-BV PET was not analyzed in this dataset as response criteria were nonstandardized.
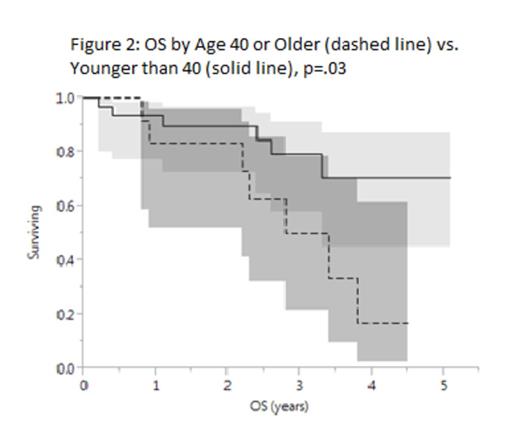
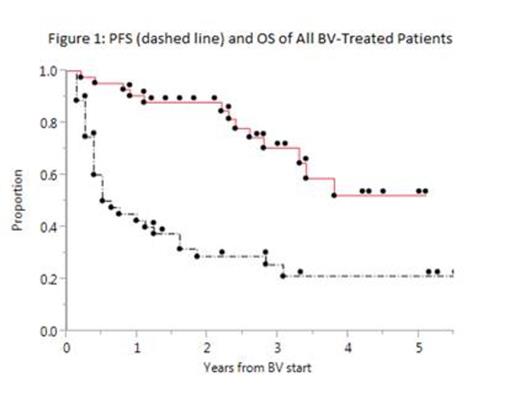
BV was administered for a median 6 cycles (range 2-20). Median PFS after BV was 6 mo. (Figure 1) with 4 pts having PFS >4 yrs. At 31 mo. median follow-up, 71% of pts were alive with no plateau in the survival curve. On univariate analysis, age 40 or older at time of BV predicted inferior PFS (p=.03) and inferior OS though 95% confidence intervals were wide (OS by age: Figure 2, p=.02). HR for death for pts age 40 or older was 4 (98% CI .03-2.3, p=.05). No other factor predicted PFS or OS.
Among 29 pts who failed BV, OS was 3.4 yrs. 40 chemotherapy regimens were given with 11 responses. Five of 11 pts responded to bendamustine, but median time to progression was 4 mo. Two of 4 responded to gemcitabine as did 3/8 receiving platinum chemotherapy.
Conclusions: In this cohort of rel/ref HL pts treated with BV, PFS was 6 mo. overall and inferior among pts 40 yrs or older. OS was also worse in this group, although confidence intervals were wide in both univariate analyses. We confirm and expand upon prior data showing features predicting outcomes in HL after chemotherapy do not clearly apply after BV; and that most pts progress <1 year after single-agent BV with a few durable remissions. Salvage chemotherapy after BV has transient efficacy. Larger studies defining clinical, biologic, and PET-based markers of outcome after BV are needed.
Off Label Use: Brentuximab is approved in HL after failure of autologous stem cell transplant; in this series, some patients received Brentuximab before or when ineligible for an autologous stem cell transplant.. Gopal:Seattle Genetics: Consultancy, Honoraria, Research Funding, Speakers Bureau; Takeda: Honoraria, Research Funding, Speakers Bureau; Janssen: Research Funding; Pfizer: Consultancy, Research Funding; BMS: Research Funding; Gilead: Research Funding; Spectrum: Research Funding; Teva: Research Funding. Shustov:Seattle Genetics, Inc.: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal