Abstract
Purpose
Extranodal natural killer/T-cell lymphoma, nasal type (ENKTL), is a highly aggressive non-Hodgkin lymphoma (NHL) with poor prognosis. For localized upper aerodigestive tract (UADT) cases, radiotherapy alone or chemotherapy plus radiotherapy yields relatively favorable prognosis. In contrast, historically, cases with advanced-stage disease or involving the extra-upper aerodigestive tract (extra-UADT) are primarily treated with anthracycline-containing chemotherapy and result in poor long-term survival due to high expression of p-glycoprotein In addition, for localized UADT disease, the overall treatment failure rate is 30%-40%, with most of the relapses being distant metastases[1-2]. Therefore, effective chemotherapeutic regimens are needed for the cases metastatic UADT and extra-UADT disease.
Some studies have reported the efficacy of L-asparaginase in patients with ENKTL. The use of the SMILE (steroid, methotrexate, ifosfamide, L-asparaginase, and etoposide) regimen was limited by a high rate of hypersensitivity reactions and severe myelosuppression[3-5]. Our previous study has demonstrated the high activity and low toxicity of gemcitabine-based regimens in patients with peripheral T cell lymphoma [6]. In this study, we aim to assess the efficacy and safety of gemcitabine-based regimens in ENKTL patients.
Patients and methods
We retrospectively reviewed 54 patients with ENKTL who received gemcitabine-based regimens between January 2008 and December 2013. 34 newly-diagnosed patients and 20 refractory/relapsed patients were enrolled. There were 27 cases of early-stage and 27 cases of advanced-stage disease. GDP (gemcitabine intravenously 1250mg/m2 on days 1 and 8; cisplatin 25mg/m2 intravenously on days 1-3; and dexamethasone 20mg/d orally on days 1-4 and days 11-14) was given to 35 patients, and P-GDP (pegaspargase 2500U/m2 intramuscularly on day 8, gemcitabine, dexamethasone, and cisplatin) given to 19 patients. The regimens were administered in 21--day cycles. Radical involved-field radiotherapy or palliative radiotherapy was implemented as necessary.
Results
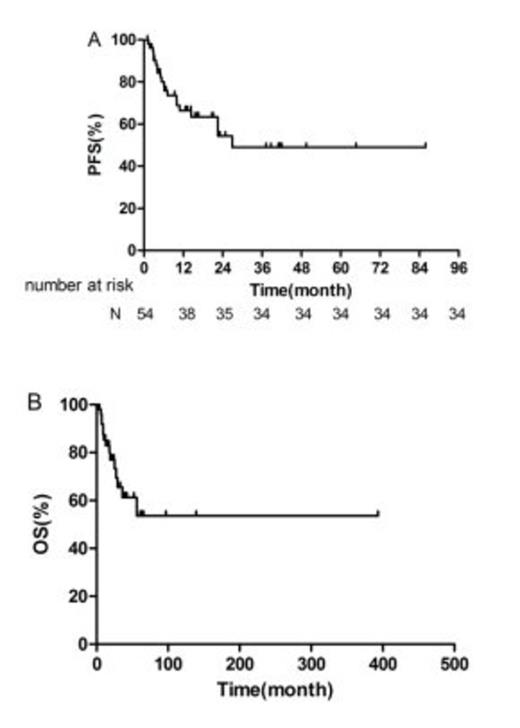
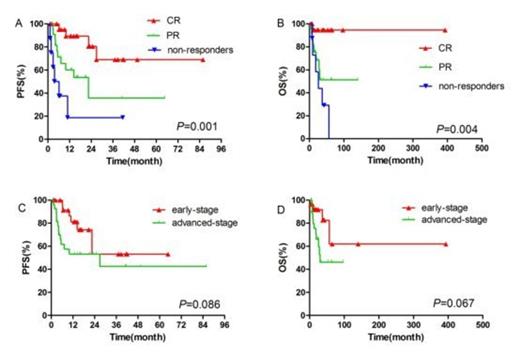
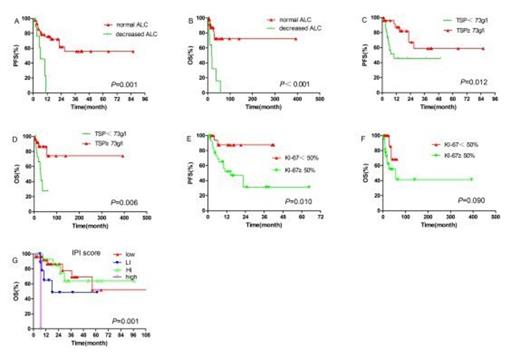
A total of 237 cycles of treatment were administered and the median number of cycles per patient was 4 (range 2-8). 24 of the 27 early-stage patients and 4 of the 27 advanced-stage patients received radiotherapy. 2 patients received hematologic stem cell transplantation as consolidation treatment. The complete-remission rate, partial-remission rate, stable-disease rate and progressive-disease rate were 42.6% (23/54), 42.6% (23/54), 11.1% (6/54), and 3.7% (2/54), respectively. Higher overall response-rate was observed in newly diagnosed patients compared to refractory/relapsed patients (94.1% vs 70.0%, P = 0.044), and in patients with normal absolute lymphocyte count (ALC) than in patients with decreased ALC (93.2% vs 50.0%, P = 0.003). After a median follow-up of 17.6 months (range 2-96.8), the 1-year and 2-year overall-survival (OS) rates for all patients were 85.3% and 76.9%; the 1-year and 2-year progression-free survival (PFS) rates for the whole cohort were 66.5% and 54.4%. Early-stage patients tended to achieve better PFS and OS than advanced-stage patients (1-year PFS: 81.2% vs 53.1%, P = 0.086, 1-year OS: 91.7% vs 79.6%, P = 0.067). In univariate analysis, chemotherapy response, KI-67 proliferation index, ALC, and total serum protein (TSP) were found to be significant prognostic factors for PFS; chemotherapy response, IPI score, ALC, and TSP were significant prognostic factors for OS. The incidence of grade 3 and 4 adverse events was as follows: neutropenia (38.9%), thrombocytopenia (25.9%). Nausea and vomiting were common non-hematological toxicities. No grade 3 or 4 non-hematological toxicities or allergic reactions were observed. Overall, both regimens (GDP and P-GDP) were well tolerated with a substantially lower rate of acute toxicities than current treatments[3-5].
Conclusion
Our clinical trial has demonstrated the high efficacy and low toxicity-profile of gemcitabine-based regimens in patients with ENKTL. Further research is warranted to elucidate its value as adjuvant chemotherapy in early-stage ENKTL patients.
[1] Cancer,2011,117(22):5203-11.
[2] Cancer,2004,100(2):366-75.
[3] Cancer Science,2008,99(5):1016-20.
[4] Journal of Clinical Oncology,2011,29(33):4410-16.
[5] Blood,2012,120(15):2973-80.
[6] Medical Oncology,2013,30(1):1-6.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal