Abstract
Background:
The LYM-3002 study compared the efficacy and safety of frontline VR-CAP (n=243) vs R-CHOP (n=244) in newly diagnosed MCL pts who were ineligible or not considered for autologous stem cell transplantation. VR-CAP substitutes bortezomib for vincristine in the standard R-CHOP regimen. LYM-3002 met its primary endpoint, demonstrating a 59% improvement in progression-free survival (PFS) per independent radiology review committee (IRC) with VR-CAP vs R-CHOP (median 24.7 vs 14.4 mos; HR 0.63 [0.50, 0.79]; p<0.001) and a 96% improvement in PFS per investigator (INV) (HR 0.51; p<0.001). Significant and clinically relevant improvements in secondary efficacy endpoints were also demonstrated with VR-CAP, including a more-than-doubling of median duration of complete response (CR), and a doubling of median treatment-free interval. In addition, 4-yr overall survival (OS) rates were 10% higher with VR-CAP (64.4% vs 53.9%). These findings were accompanied by additional but expected and manageable toxicities (Cavalli F, et al. ASCO 2014, Abs 8500; Robak T, et al. EHA 2014, Abs S1345). Reflecting the real-life situation, the protocol allowed for inclusion of pts not considered for transplantation for non-medical reasons (e.g. pt refusal, financial affordability). This post-hoc analysis of the LYM-3002 study evaluated the efficacy and safety of VR-CAP vs R-CHOP in a subgroup of 80 pts who were aged <60 yrs and without medical reasons for transplant ineligibility per the sponsor’s medical monitor assessment.
Methods:
Adults with measurable stage II–IV MCL and ECOG PS 0–2 were randomized 1:1 (stratified by IPI score and disease stage) to 6–8 x 21-d cycles of rituximab 375 mg/m2, cyclophosphamide 750 mg/m2, doxorubicin 50 mg/m2, all IV d 1, and prednisone 100 mg/m2 PO d 1–5, plus bortezomib 1.3 mg/m2 IV d 1, 4, 8, 11 (VR-CAP) or vincristine 1.4 mg/m2 (max 2 mg) IV d 1 (R-CHOP). Endpoints of this analysis were PFS, rates of CR/unconfirmed CR (CR/CRu), duration of CR/CRu, OS, and safety (adverse events [AEs]; NCI-CTCAE v3.0). PFS and response were assessed by IRC and by INV per International Lymphoma Workshop Response Criteria. PFS and OS were estimated by Kaplan-Meier methodology. The Cochran-Mantel-Haenszel Chi-squared test was used for response rate comparisons.
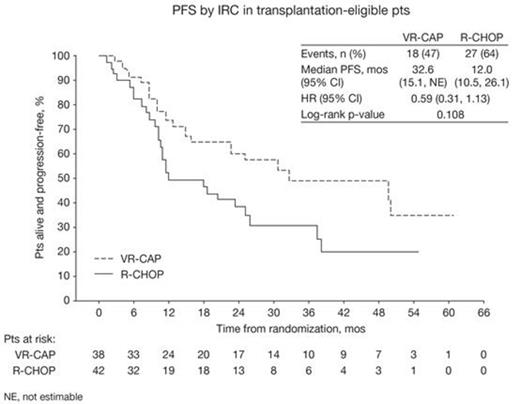
Results:
The 80 pts (38 VR-CAP; 42 R-CHOP) included in this analysis were enrolled in countries in the EU (11%), North America region (1%), and Rest of World (88%; mainly China [36%] and Russia [23%]). Median age was 54 yrs (34–59). 78% of pts were male, 53%/48% were Asian/White, 58%/38%/5% had ECOG PS 0/1/2, 8%/33%/60% had stage II/III/IV MCL at diagnosis, and 56%/31%/11%/1% had IPI score 0–1/2/3/4–5. 76%/16%/8% had MIPI low-/int-/high-risk status (54%/28%/18% MIPIb low-/int-/high-risk) and 38%/62% had Ki-67-high/-low MCL. Baseline characteristics and stratification factors were generally similar between arms. Pts completed a median of 6 cycles. With VR-CAP vs R-CHOP, median PFS by IRC was 32.6 vs 12.0 mos (HR 0.59; p=0.108; Figure) and by INV was 42.6 vs 20.6 mos (43 [54%] events; HR 0.54 [0.28, 1.03]; p=0.057). In response-evaluable pts (36 VR-CAP; 41 R-CHOP), rates of CR/CRu by IRC (bone marrow and LDH verified) were 67% vs 39% (OR 3.7 [1.3, 10.4]; p=0.012) and by INV were 50% vs 29% (OR 2.2 [0.8, 5.9]; p=0.126). Median duration of CR/CRu by IRC (24 vs 16 pts evaluable) was 45.9 vs 28.6 mos, and by INV (18 vs 12 pts evaluable) was 48.0 mos vs not reached (NR). Median OS with VR-CAP vs R-CHOP was NR vs 47.3 mos (24 [30%] events; HR 0.81 [0.33, 1.96]; p=0.634). 4-yr OS rates were 74.1% (54.0%, 86.5%) vs 48.7% (28.5%, 66.2%), respectively. 95% vs 81% (VR-CAP vs R-CHOP) of pts had grade ≥3 AEs which were mainly hematologic and included neutropenia (89% vs 67%), leukopenia (57% vs 31%), thrombocytopenia (59% vs 0), lymphopenia (22% vs 12%), anemia (16% vs 14%), and febrile neutropenia (8% vs 12%). Rates of serious AEs (19% vs 19%), discontinuations due to AEs (2 vs 1 pt), and grade 5 AEs (2 vs 2 pts) were similar between arms.
Conclusions:
In younger pts not considered for transplantation, VR-CAP appears to provide a benefit vs R-CHOP in terms of PFS, CR/CRu rate, duration of CR/CRu, and OS, consistent with findings in the overall LYM-3002 pt population. Notably, a 67% CR/CRu rate by IRC with a median CR/CRu duration of 45.9 mos was achieved with VR-CAP. Further research is needed to determine how these results might be improved by consolidation with transplantation and maintenance therapy.
Drach:Janssen: Consultancy, Honoraria; Takeda: Consultancy, Honoraria. Off Label Use: The proteasome inhibitor bortezomib is approved in the US for the treatment of multiple myeloma and for the treatment of patients with mantle cell lymphoma who have received at least one prior therapy. In the present study, bortezomib is being investigated in combination with immunochemotherapy for previously untreated patients with mantle cell lymphoma, an indication for which it is currently not approved.. Belch:Janssen: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Celgene: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Farber:Alexion: Equity Ownership, Speakers Bureau; Genentech: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Gilead: Speakers Bureau; Janssen/Pharmacyclics: Speakers Bureau; Seattle Genetics: Speakers Bureau; Leukemia Lymphoma Society NJ Chapter: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Bosly:Amgen: Speakers Bureau; Roche: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Zaucha:Roche: Honoraria; Amgen: Honoraria; Janssen: Honoraria; Celgene: Honoraria; Takeda: Honoraria. Robak:Janssen Research & Development: Consultancy, Research Funding. Pei:Janssen: Employment. Rooney:Janssen: Employment; Johnson & Johnson: Equity Ownership. van de Velde:Janssen: Employment; Johnson & Johnson: Equity Ownership. Cavalli:Mundipharma: Research Funding; Pfizer: Research Funding; Roche: Research Funding; Takeda: Consultancy; Novartis: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal