Abstract
Background:
LYM-3002 was a global study evaluating the efficacy and safety of frontline VR-CAP vs R-CHOP in newly diagnosed MCL pts who were not considered for hematopoietic stem cell transplantation. 487 pts (243 VR-CAP, 244 R-CHOP) were enrolled in 28 countries in the EU, North/South America, and Rest of World. VR-CAP was associated with significantly longer progression-free survival (PFS) by independent radiology review committee (IRC) vs R-CHOP (median 24.7 vs 14.4 mos; hazard ratio [HR] 0.63 [0.50, 0.79]; p<0.001). Secondary efficacy endpoints including complete response (CR)/unconfirmed CR (CR/CRu) rates by IRC (odds ratio [OR] 1.69), time to progression by IRC (HR 0.58), time to next therapy (TTNT; HR 0.50), treatment-free interval (TFI; HR 0.50), overall survival (OS; HR 0.80), and 4-yr OS rates (64.4% vs 53.9%) were also consistently improved with VR-CAP vs R-CHOP, with additional but expected and manageable toxicity (Cavalli et al. ASCO 2014, Abs 8500; Robak et al. EHA 2014, Abs S1345). VR-CAP has not previously been evaluated in Japanese pts with newly diagnosed B-cell lymphoma. This sub-analysis of LYM-3002 evaluated the efficacy and safety of VR-CAP vs R-CHOP in the cohort of Japanese MCL pts enrolled in the study. While the traditional drug development process in Japan requires a small separate phase 1/2 study in Japanese patients to confirm international study results, here a pre-planned Japanese subgroup was included in the larger phase 3 study.
Methods:
Adults with measurable stage II–IV MCL and ECOG PS 0–2 were randomized 1:1 (stratified by International Prognostic Index [IPI] score and disease stage) to 6–8 × 21-d cycles of rituximab 375 mg/m2, cyclophosphamide 750 mg/m2, doxorubicin 50 mg/m2, all IV d 1, and prednisone 100 mg/m2 PO d 1–5, plus bortezomib 1.3 mg/m2 IV d 1, 4, 8, 11 (VR-CAP) or vincristine 1.4 mg/m2 (max 2 mg) IV d 1 (R-CHOP). Response was assessed by IRC and by investigator (INV) per International Lymphoma Workshop Response Criteria. Time-to-event outcomes were estimated by Kaplan-Meier methodology. The Cochran-Mantel-Haenszel Chi-squared test was used for response rate comparisons. Adverse events (AEs) were graded per NCI-CTCAE v3.0.
Results:
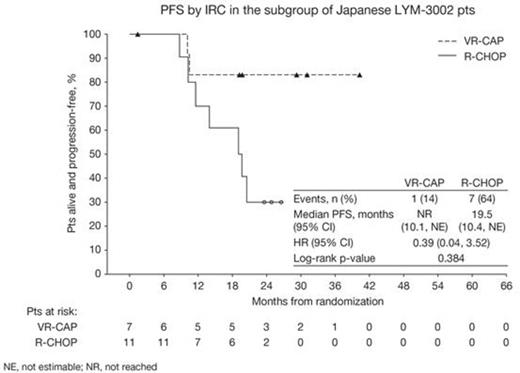
18 Asian pts with centrally confirmed MCL (7 VR-CAP, 11 R-CHOP) were enrolled in Japan. For VR-CAP vs R-CHOP, pts’ median age was 70 (64–74) and 70 (66–82) yrs; 6 vs 11 pts were aged >65 yrs. 4 vs 8 pts were male, 4/2/1 vs 8/3/0 pts had ECOG PS 0/1/2, 0/2/5 vs 1/1/9 pts had stage II/III/IV disease at diagnosis, and 0/3/3/1 vs 1/3/6/1 pts had IPI score low (0–1)/low–intermediate (2)/high–intermediate (3)/high (4–5). All pts completed ≥6 cycles, except for 1 pt on VR-CAP who discontinued after 2 cycles (grade 2 hypertension). After a median follow-up of ~33 mos, median PFS by IRC with VR-CAP vs R-CHOP was not reached (NR) vs 19.5 mos (1/7 vs 7/11 progression/death events; HR 0.39 [0.04, 3.52]; p=0.384; Figure) and by INV was NR vs 16.2 mos (2/7 vs 9/11 progression/death events; HR 0.46 [0.09, 2.45]; p=0.351). In 17 response-evaluable pts (7 VR-CAP vs 10 R-CHOP), rates of CR/CRu by IRC (bone marrow and LDH-verified) were 71% vs 50% (OR 3.0 [0.15, 59.89]; p=0.495) with median duration of CR/CRu NR vs 16.6 mos, and overall response rates (ORR; CR+CRu+partial response [PR]) were 100% in both arms. Median TFI was NR vs 19.1 mos (HR 0.71 [0.13, 3.89]; p=0.688) and median TTNT was NR vs 24.3 mos (HR 0.71 [0.13, 3.89]; p=0.688), respectively. After only 1 death (R-CHOP arm), median OS for VR-CAP and R-CHOP were not estimable (NE). 3-yr OS rates were 100.0% (NE, NE) vs 80.0% (20.4%, 96.9%). All pts experienced at least one grade ≥3 AE, most commonly neutropenia (100% VR-CAP vs 100% R-CHOP), thrombocytopenia (71% vs 0), febrile neutropenia (43% vs 45%), lymphopenia (43% vs 27%), leukopenia (29% vs 27%), and anemia (0 vs 27%). No grade ≥3 bleeding AEs were reported and grade ≥3 infection AEs occurred in only 1 pt on VR-CAP (herpes zoster infection). Peripheral neuropathy rates were 71% vs 45% (grade ≥3: 14% vs 9%). 1 R-CHOP pt experienced serious AEs (bile duct stone, femur fracture). There were no on-treatment deaths.
Conclusions:
Results from this cohort of Japanese pts enrolled in LYM-3002, showing improved efficacy in favor of VR-CAP with additional but manageable toxicity, appear consistent with the findings in the overall study population. VR-CAP could be considered as an alternative frontline treatment option for newly diagnosed, transplantation-ineligible MCL pts in Japan.
Ogura:Janssen: Research Funding; Takeda: Research Funding; Chugai: Research Funding; Celgene: Research Funding; Symbio: Research Funding; Kyowahakko-Kirin: Research Funding; Pfizer: Research Funding; GlaxoSmithKline: Research Funding. Off Label Use: The proteasome inhibitor bortezomib is approved in the US for the treatment of multiple myeloma and for the treatment of patients with mantle cell lymphoma who have received at least one prior therapy. In the present study, bortezomib is being investigated in combination with immunochemotherapy for previously untreated patients with mantle cell lymphoma, an indication for which it is currently not approved.. Pei:Janssen: Employment. Rooney:Janssen: Employment; Johnson & Johnson: Equity Ownership. van de Velde:Janssen: Employment; Johnson & Johnson: Equity Ownership. Cavalli:Novartis: Consultancy; Takeda: Consultancy; Roche: Research Funding; Pfizer: Research Funding; Mundipharma: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal