Abstract

BACKGROUND: Peripheral T-cell lymphoma, not otherwise specified (PTCL-NOS), is relatively rare, accounting for only 10% to 15% of non–Hodgkin's lymphomas, and is characterized by disseminated disease, with systemic symptoms, bone marrow involvement, and extranodal disease. There is no general consensus regarding the preferred induction chemotherapy for PTCL-NOS. CHOP regimen consisting of cyclophosphamide (CPA), doxorubicin (DOX), vincristine (VCR) and prednisone (PSL) has been the most frequently employed regimen for PTCL-NOS, but the overall survival in each IPI category appears lower in patients with most PTCL-NOS than seen in diffuse large B-cell lymphoma. Pirarubicin (tetrahydropyranyladriamycin: THP), a derivative of DOX, is reportedly an anthracyclin with less cardiotoxicity than DOX, because the cardiac sympathetic dysfunction and cardiac mitochondrial damage were less common with THP than those with doxorubicin. We previously reported THP-COP regimen consisting of THP, CPA, VCR, and PSL produced resulted equivalent to CHOP regimen regarding efficacy and safety in patients with aggressive non-Hodgkin's lymphoma (Tsurumi H et al. JCRCO 2004). In addition The Japanese Clinical Study Group of THP Lymphomas in the Elderly reported that T-cell lymphomas had a significantly better response to THP-COP than CHOP, and no such difference was observed in B-cell lymphoma. Therefore, we conducted a retrospective analysis to confirm the efficacy of THP-COP in the treatment of PTCL-NOS.
PATIENTS AND METHODS: The study protocol employed a retrospective, consecutive entry design. We retrospectively analyzed 61 patients with PTCL-NOS who had received THP-COP or CHOP in 5 institutes of Gifu Hematology Study Group between December 1995 and March 2013. A diagnosis of PTCL-NOS was confirmed histrogically according to the World Health Organization classification. We exclude patients who had previously treated with any chemotherapy for lymphoma, and were diagnosed as Adult T-cell leukemia/lymphoma or angioimmunoblastic T-cell lymphoma. CHOP regimen composed CPA (750 mg/m2), DOX (50 mg/m2), VCR (1.4 mg/m2, maximal dose 2.0 mg) and PSL (100mg/body, administered for 5 days). In THP-COP regimen, THP (50 mg/m2) was used instead of DOX. These regimens were performed every 14 to 21 days. Thirty patients received THP-COP, 31 received CHOP. There were no significant differences in known prognostic factors include the international prognostic index (IPI) and prognostic index for T-cell lymphoma (PIT) between two groups. The median cycles of treatment were 6 in both groups. The median follow-up times were 25 and 19 months, respectively.
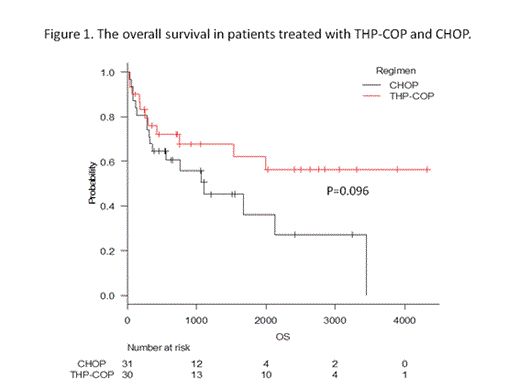
RESULT: Complete remission (CR) rates in the patients with THP-COP and CHOP were 53% and 55% (P=0.906), 3-yr overall survival (OS) rate 68% and 51% (P=0.096), and 3-yr progression free survival (PFS) rate 46% and 27% (P=0.078), respectively. Although there were no significant differences between two groups, THP-COP regimen had a tendency of better prognosis. In patients with low IPI (Low or Low-intermediate), THP-COP had significantly better 3yr-OS (100% vs 63%, P=0.002) and 3yr-PFS (69% vs 34%, P=0.002). Same difference was observed in patients with low PIT (group1 or 2), but was not observed in patients with high IPI (High-intermediate or High) or PIT (Group3 or 4). Fatal non-hematological adverse event did not occur in both groups.
CONCLUSION: Our study showed THP-COP produced resulted equivalent to CHOP regarding efficacy and safety in patients with PTCL-NOS. Moreover, THP-COP had a tendency of better prognosis compared with CHOP. In patients with low IPI or PIT, THP-COP had significantly better prognosis. This result indicates that THP-COP is an effective and well tolerated regimen for patients with PTCL-NOS, and might be translated into improving survival in comparison with CHOP. Further large study is needed to clarify the difference between the two regimens.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal