Abstract
Context: Primary testicular lymphoma (PTL) is an aggressive form of extranodal lymphoma almost always of B-cell lineage. The data regarding prognostic indicators and appropriate treatment regimens in patients with PTL, especially in the era of rituximab-cyclophosphamide, hydroxydaunorubicin, vincristine and prednisone (R-CHOP) therapy are not well established.
Objective: To evaluate the clinicopathologic features and their impact on outcome in patients with primary testicular lymphoma (PTL) of B-cell lineage.
Patients and Design: We retrospectively analyzed the characteristics of 89 patients with PTL referred to our institution between September 1, 1998 and May 31, 2014. Follow up data was available for all patients. Detailed staging and therapy information were available for 69 and 63 patients, respectively. Cell of origin classification of DLBCL cases was determined by immunohistochemistry when possible.
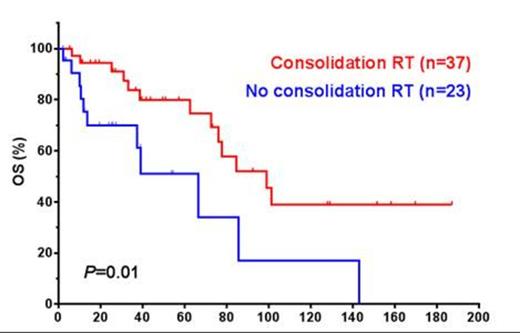
Results: The median age at diagnosis was 63.1 years (range, 2.9-96.2). Patients presented with stage I (26/66, 40%), stage II (14/66, 21%), stage III (4/66, 1%) and stage IV (25/66, 38%) disease, respectively. Histologic variants included: diffuse large B-cell lymphoma (DLBCL) (n=82), Burkitt lymphoma (BL) (n=2), B-cell lymphoma, unclassifiable, with features intermediate between DLBCL and BL (n=4) and blastoid mantle cell lymphoma (n=1). Twenty-six (40%) patients had involvement of spermatic cord, epididymis and paratesticular tissues and 16 (18%) tumors showed coagulative tumor necrosis. In 31 cases DLBCL was stratified into germinal center B-cell-like (n=16, 52%) and non-germinal center B-cell-like (n=15, 42%). Six of 36 (16%) tumors analyzed showed CD5 expression by immunohistochemistry and/or flow cytometry. The median Ki-67 proliferation index was 80% in 24 cases analyzed (range, 40-100%). Four of 16 cases (25%) analyzed were positive for MYC rearrangement by fluorescence in situ hybridization. Among patients with available data (n=63); all received systemic chemotherapy (41, R-CHOP, 9 CHOP and 13 other regimens) +/- prophylactic intrathecal chemotherapy (n=48) and/or consolidation involved field radiation therapy (n=37, 23 patients with stage I/II and 12 patients with stage III/IV disease). Response to therapy was documented in 61 patients; 85% achieved complete remission, 7% partial remission and 8% had progressive disease (PD). Recurrent and/or PD were seen in 28 of 65 (43%) patients with available data. The most frequent sites of recurrence included lymph nodes (23%), soft tissues (19%), leptomeninges/brain (19%) and contralateral testicle (13%). The median overall survival (OS) and progression free survival (PFS) were 84.7 and 55.7 months, respectively. Advanced Ann Arbor stage (p=0.05, PFS), involvement of ≥2 extranodal sites (p=0.05, OS), elevated serum LDH level (p=0.013 and 0.026, OS and PFS respectively), and International Prognostic Index ≥2 (p=0.04, OS) were significantly associated with poorer outcome. Administration of prophylactic intrathecal chemotherapy (p=0.0017 and 0.012, OS and PFS, respectively) and consolidation radiation therapy (Figure 1, p=0.01 and 0.033, OS and PFS, respectively) were significantly associated with better outcome by univariate analysis. The association of consolidation radiation therapy with outcome was diminished in patients with stage III and IV disease. There was no significant association between different chemotherapy regimens and outcome, although patients who received rituximab showed a trend for better OS and PFS. Microscopic involvement of spermatic cord, epididymis and paratesticular tissue was associated coagulative necrosis (p= 0.04) and poorer outcome (p=0.018 and 0.023, OS and PFS respectively). Non-DLBCL histology type was significantly associated with nodal involvement at presentation (p=0.05). We observed a trend for shorter PFS in patients whose tumors had a Ki-67 proliferation index of ≥90% (p=0.2). There was no significant association between GCB vs. non-GCB subtypes, CD5 expression or MYC gene rearrangement and outcome.
Conclusions: Our results indicate that PTL of B-cell lineage represents a distinctive group of extranodal lymphomas with unique clinical, biological and prognostic features. The data further demonstrate the beneficial therapeutic impact of prophylactic intrathecal chemotherapy and consolidation radiation therapy in patients with PTL.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal