Abstract
Background: Human Immunodeficiency Virus (HIV) infection is associated with an increased risk of classical Hodgkin (cHL) and non-Hodgkin lymphomas (NHL). In the combined antiretroviral therapy (cART) era, while the incidence of NHL has declined, HL incidence has remained stable. In comparison to their HIV-negative counterpart, HIV-associated HLs present frequent high-risk characteristics: 1- mixed cellularity histological subtype, 2- advanced stage, presence of B symptoms and higher International Prognostic Score (IPS).
Methods: The national prospective cohort of HIV-related lymphomas (ANRS CO16 Lymphovir cohort sponsored by Inserm-ANRS) enrolled adult patients in 40 centers since July 2008. Pathological materials were centralized and reviewed. Diagnoses were based on World Health Organization criteria. Patients have been followed every 6 months during 5 years. HIV HL patients were compared to a series of HIV-negative adult patients consecutively diagnosed with HL during the same period in Paris Saint-Louis hospital.
Results: Among 159 HIV-infected patients, 68 (43%) were diagnosed with cHL. Mixed cellularity (MC) subtype was predominant (n=42), followed by nodular sclerosing (11). Fifteen cases could not be subclassified mainly because of small needle biopsies. Median age was 44 years (ranging from 20 to 65), male/female ratio was 5.8. Most patients (75%) had advanced clinical stage (III/IV). HIV infection had been diagnosed for a median of 13 years (maximum of 26 years) before the diagnosis of cHL. Median CD4 T-cell count was 380/μl (range 37-1742) and plasma HIV RNA was < 50 in 78% of the patients. All except one patient had been treated with cART prior to cHL diagnosis. They were all treated with cART after diagnosis. Front-line chemotherapy with standard anthracyclin based regimen (ABVD) was given to 64 out of 68 patients, BEACOPP in 4 patients. ABVD was followed by radiotherapy in limited stages. Five patients died from early disease progression (n=2), sepsis during chemotherapy (1) and after relapse (2).
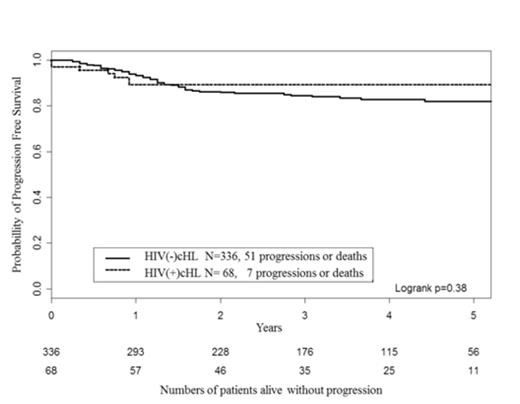
Two-year overall survival and Progression Free Survivals (PFS) were 94% [95%CI 88, 100] and 89% [82, 97], respectively. The only factor associated with PFS was age with a relative risk of 8.09 [0.97; 67.18] above 45 years (Table). IPS and CD4 count were not significantly associated with PFS. In comparison with HIV-negative patients, patients with HIV infection displayed higher risk features for all prognostic factors of HL (older age, male predominance, MC subtype, more advanced stages, lower lymphocytic counts). Treatment of HIV-negative patients was similar to HIV-positive patients: ABVD (79%), BEACOPP (21%), with addition of radiotherapy in limited stages. Overall, the outcomes of the HIV-infected patients did not differ significantly from those of HIV-negative patients (Figure).
Conclusion : Although high risk features still predominate in HIV-HL, the prognostic of these patients has markedly improved in the recent cART era. We report 2-years OS and PFS of 94% and 89%, respectively, in patients mainly treated with cART and ABVD. The outcome of HL in HIV infected patients now resembles to those of non HIV infected patients.
Univariate analysis of Progression Free Survival in HIV associated Hodgkin Lymphoma (Cox model) (N=68)
| . | N . | Progression or Death, N . | RR . | 95 % CI . | p-value . |
|---|---|---|---|---|---|
| Gender | 0.95 | ||||
| F | 9 | 1 | 1 | ||
| M | 59 | 6 | 0.94 | [0.11 ; 7.77] | |
| Age | 0.05 | ||||
| <45 | 38 | 1 | 1 | ||
| ≥45 | 30 | 6 | 8.09 | [0.97 ; 67.18] | |
| Ann-Arbor stage | 0.77 | ||||
| I-II | 16 | 1 | 1 | ||
| III | 20 | 2 | 1.54 | [0.14; 17.00] | |
| IV | 32 | 4 | 2.09 | [0.23; 18.72] | |
| Hemoglobin, g/dL | 0.23 | ||||
| ≥10.5 | 44 | 3 | 1 | ||
| <10.5 | 24 | 4 | 2.5 | [0.56 ; 11.16] | |
| Leucocytes, x109/L | 0.14 | ||||
| <15 | 66 | 6 | 1 | ||
| ≥15 | 2 | 1 | 4.94 | [0.59 ; 41.05] | |
| Lymphocytes, x109/L* | 0.49 | ||||
| ≥0.6 | 53 | 5 | 1 | ||
| <0.6 | 12 | 2 | 1.79 | [0.35 ; 9.21] | |
| Albumin, g/L** | 0.40 | ||||
| >=40 | 18 | 1 | 1 | ||
| <40 | 42 | 6 | 2.50 | [0.30 ; 20.76] | |
| IPS ** | 0.32 | ||||
| 0-2 | 19 | 1 | 1 | ||
| 3-7 | 41 | 6 | 2.91 | [0.35 ; 24.20] | |
| CD4 cell count, x109/L *** | 0.52 | ||||
| > 0.2 | 45 | 4 | 1 | ||
| ≤0.2 | 21 | 3 | 1.64 | [0.37 ; 7.32] |
| . | N . | Progression or Death, N . | RR . | 95 % CI . | p-value . |
|---|---|---|---|---|---|
| Gender | 0.95 | ||||
| F | 9 | 1 | 1 | ||
| M | 59 | 6 | 0.94 | [0.11 ; 7.77] | |
| Age | 0.05 | ||||
| <45 | 38 | 1 | 1 | ||
| ≥45 | 30 | 6 | 8.09 | [0.97 ; 67.18] | |
| Ann-Arbor stage | 0.77 | ||||
| I-II | 16 | 1 | 1 | ||
| III | 20 | 2 | 1.54 | [0.14; 17.00] | |
| IV | 32 | 4 | 2.09 | [0.23; 18.72] | |
| Hemoglobin, g/dL | 0.23 | ||||
| ≥10.5 | 44 | 3 | 1 | ||
| <10.5 | 24 | 4 | 2.5 | [0.56 ; 11.16] | |
| Leucocytes, x109/L | 0.14 | ||||
| <15 | 66 | 6 | 1 | ||
| ≥15 | 2 | 1 | 4.94 | [0.59 ; 41.05] | |
| Lymphocytes, x109/L* | 0.49 | ||||
| ≥0.6 | 53 | 5 | 1 | ||
| <0.6 | 12 | 2 | 1.79 | [0.35 ; 9.21] | |
| Albumin, g/L** | 0.40 | ||||
| >=40 | 18 | 1 | 1 | ||
| <40 | 42 | 6 | 2.50 | [0.30 ; 20.76] | |
| IPS ** | 0.32 | ||||
| 0-2 | 19 | 1 | 1 | ||
| 3-7 | 41 | 6 | 2.91 | [0.35 ; 24.20] | |
| CD4 cell count, x109/L *** | 0.52 | ||||
| > 0.2 | 45 | 4 | 1 | ||
| ≤0.2 | 21 | 3 | 1.64 | [0.37 ; 7.32] |
RR: Relative Risk
CI: Confidency interval
*: 3 missing values
**: 8 missing values
***: 2 missing values
Median follow-up:
HIV(-) cHL: 37 months (IQR=37)
Lymphovir: 33 months (IQR=34)
2- year PFS:
HIV(-) cHL: 0.86 CI95%=[ 0.82, 0.90 ]
Lymphovir: 0.89 CI95%=[ 0.82, 0.97 ]
Progression free survival (PFS) of HIV associated Hodgkin’s Lymphoma (N= 68) compared with HIV negative patients (N=336)
Progression free survival (PFS) of HIV associated Hodgkin’s Lymphoma (N= 68) compared with HIV negative patients (N=336)
IQR: Interquartile range
CI: Confidency interval
Brice:Seattle Genetics, Inc.: Research Funding; Takeda Pharmaceuticals International Co.: Honoraria, Research Funding; Roche: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal