Abstract

INTRODUCTION: Transfusion-associated graft-vs-host disease (TA-GVHD) is a rare and often fatal complication of transfusion of cellular blood products. The relative contributions of product and donor factors to the risk of TA-GVHD remain uncertain.
METHODS: Systematic review of all reported cases of TA-GVHD in the published literature prior to Oct 2013, without language restrictions. Cases attributed to granulocyte transfusions, passenger lymphocyte syndrome, or GVHD following stem-cell transplant (unless traced to blood components rather than the graft) were excluded. Data collected included patient demographics and health information, details of transfusion event(s) and blood component(s), clinicolaboratory features of the TA-GVHD presentation and outcome, and results of human leukocyte antigen (HLA) and chimerism studies. HLA typing was evaluated where reported for both donor (product) and recipient at either class I or class II loci. Donor/recipient pairs were categorized as D=0 when there were no identified donor HLA antigens foreign to the recipient, or D>0 when donor cells contained one or more HLA antigens not found in the recipient. This classification applied separately to HLA class I and class II loci for each case.
RESULTS: After removing duplicates, 2130 citations discovered by the search were examined by two independent reviewers, with 394 identified as publications of interest for complete review. An additional 21 publications were found from the initial review, for a total of 415 publications. Of these, 195 publications described 348 unique cases for inclusion.
Component: The component implicated in TA-GVHD was identified in 248 (71%) cases: Red cells (RBC) in 132 (38%); whole blood (WB) in 92 (26%); platelets in 20 (6%); buffy- coat product in 2 (0.6%); and plasma and plasma-reduced blood in one case each. In 100 (29%) cases, the blood component was either not specified or not identified among several potentially responsible components.
Storage: Component storage time was reported in 158 (45%) cases. Of these, the implicated product was either described as “fresh” or </=10 days old in 148 (94%). 10 (6%) cases reported a storage time >10 days (maximum 14 days).
Related donor: In 63 cases, the donor was either related (n=61) or deliberately HLA-matched (n=2) to the recipient, while in 113 cases the donor was unrelated. The remaining cases either reported a “possible” related donor or did not report the donor-recipient relationship.
Leukoreduction/Irradiation: Leukoreduction status was reported in 135 (39%) cases. Of these, the implicated product was leukoreduced in 23 (17%) (10 bedside, 2 pre-storage, 11 not specified). The product was irradiated in 9 cases.
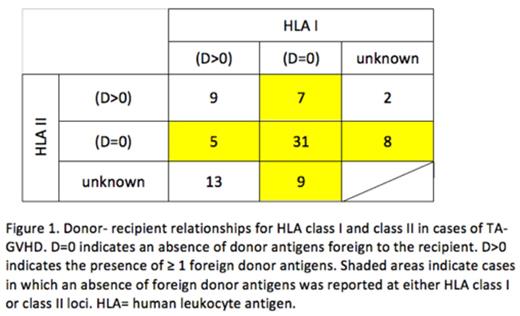
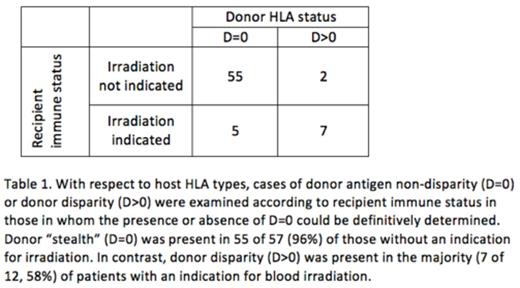
HLA: HLA typing of recipient and donor, by serological or molecular techniques, was available for 84 cases (74 cases Class I, 62 Class II). Among patients with HLA data available, 20 (24%) had an underlying diagnosis warranting irradiation by current standards, while 64 (76%) did not. The category of D=0 was found in 47 (64%) of cases with reported class I typing; 44 (71%) of cases with reported class II typing; and 60 (71%) overall (Figure 1). There were 9 cases in which the category of D=0 could be ruled out for both HLA I and II. In the remaining 15 cases, the category of D=0 at either HLA I or II could not be definitively ruled in or out based on reported data. When considering those in whom the presence or absence of D=0 could be definitely determined, while D=0 at either HLA class I or class II was present in 55 of 57 (96%) of recipients without an indication for blood component irradiation, D=0 was present in only 5 of 12 (42%) of recipients with an indication for irradiation, p< 0.0001 (Table 1).
CONCLUSIONS: The most common components implicated in TA-GVHD were WB and RBC. Most units were non-leukoreduced and stored for <10 days. Most cases of TA-GVHD occurred in recipients without a standard indication for irradiation. The absence of a foreign donor antigen at either HLA class I or class II occurred in a large majority of cases and was significantly more common in TA-GVHD among recipients without an indication for irradiation compared with those in whom irradiation would be indicated, suggesting that this donor-recipient relationship is the predominant risk factor in the development of TA-GVHD. Policies for irradiating cellular blood components based solely on the diagnosis of the recipient may fail to address all relevant risk factors for TA-GVHD.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal