Abstract
Introduction
A critical task for the clinician is to ensure that the benefits of a given drug outweigh its harms. Antibiotic-induced thrombocytopenia presents a particularly thorny dilemma in which the dual problems of infection and antibiotic-induced bleeding risk must be effectively balanced. Though previous research has assessed hemorrhage-associated mortality in the context of general drug-induced thrombocytopenia (Pedersen-Bjergaard et al European Journal of Clinical Pharmacology 1997), we sought to evaluate those risks specifically in the context of antibiotic-induced thrombocytopenia and to compare hemorrhage-associated mortality to all-cause mortality in this population.
Methods
We report an ongoing, single center observational cohort study at an urban community teaching hospital seeking to compare the risk of hemorrhage associated mortality with that of non-hemorrhage associated mortality in antibiotic-induced thrombocytopenia. Mortality is considered hemorrhage associated if an active bleed is noted within one week prior to death. Thrombocytopenia is classified as mild, moderate, or severe depending on platelet nadir: “mild” is a decline to <150,000/µL, “moderate” is a decline to <50,000/µL, and “severe” is a decline to <20,000/µL. To qualify as antibiotic-induced, the platelet reduction must have begun between two and ten days after antibiotic induction (Visentin et al Hematology/Oncology Clinics of North America 2008). We began examining electronic and paper medical records in June 2014; to date, 44 patients have met criteria (28 female and 16 male). Information related to demographics, comorbidities, medications, thrombocytopenia severity, clinical approach to thrombocytopenia management, hemorrhage, and mortality have been recorded and analyzed. Incidence of hemorrhage associated mortality has been compared with incidence of non-hemorrhage associated mortality and T-tests were used to evaluate the significance of the effects of thrombocytopenia severity and specific antibiotics on mortality.
Results
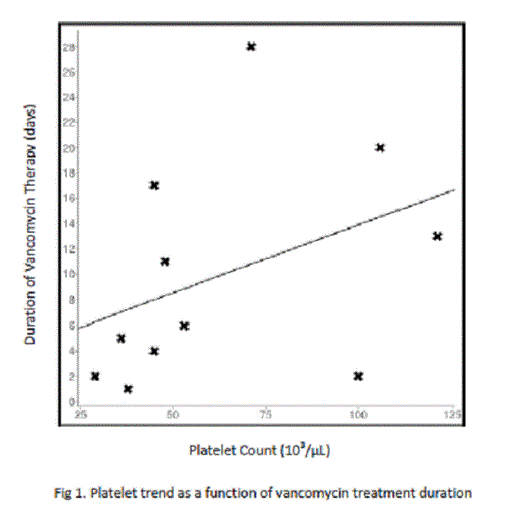
Of the 44 patients thus far evaluated, 5 expired during admission (11.36%) with 1 death being attributed to hemorrhage associated causes (worsening intracranial bleed) and 4 attributed to sepsis-related causes (2.27% v. 9.09%, respectively). Platelet nadir has been found to be lower in patients who died compared to those who lived (38,000/µL to 101,000/µL, p < 0.05), with the platelet nadir of the hemorrhage associated death found to be 36,000/µL. The association between platelet nadir and mortality incidence, however, appears to be attributable to the severity of the patient’s sepsis and not a harbinger of future bleed, as the difference in mean platelet nadir of the 3 patients who developed new-onset hemorrhage was not significantly different from those who did not (52,000/µL v. 81,000/µL, p > 0.05). Also of note was the effect of vancomycin on platelet count. Though less commonly implicated in thrombocytopenia than other antibiotics such as linezolid or piperacillin-tazobactam, vancomycin was shown to have the strongest impact on platelet count among its cohorts (mean platelet nadir: 74,000/µL compared to 104,000/µL, p = 0.03). The difference in mortality between vancomycin managed and non-vancomycin managed patients, however, has not been significant (28.6% to 7.14%, p = 0.08) which may be attributed to the tendency for platelet count to rebound with prolonged vancomycin use, as demonstrated on linear regression analysis (coefficient 0.39; Fig. 1). Similar results were found with the effect of prolonged piperacillin/tazobactam use on platelet count (coefficient 0.46; Fig. 2).
Conclusion
Antibiotic-managed patients who develop thrombocytopenia would appear to be at greater risk of death from non-hemorrhagic causes, particularly sepsis complications, than from hemorrhagic causes. Absent a severely low platelet count, history of bleed, and/or active bleed, a patient may benefit more from continuing with effective antibiotics--even putatively thrombocytopenic antibiotics--than from discontinuing them or substituting them for suboptimal antibiotics.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal