Abstract
Introduction: Each year, at least 500,000 patients will survive acute sepsis. Anemia is extremely common in sepsis survivors, further interfering with quality of life and activities of daily living. The pathogenesis of anemia of sepsis is unclear. High mobility group box 1 (HMGB1) is a necessary and sufficient inductor of persistent inflammation in sepsis survivors. After translocation form the nucleus to the cytoplasm, HMGB1 undergoes epigenetic changes in response to environmental queues. Redox state is the best well understood inducer of those modifications. In experimental sepsis, the reduced all-thiol form is the predominant form throughout the first four weeks of sepsis. We recently found that HMGB1 is crucial for the development and maintenance of anemia in sepsis in part by inducing a block in differentiation of late erythropoiesis. Here we present evidence indicating a critical role of the all-thiol HMGB1 in anemia of sepsis survivors through a CXCR4-dependent mechanism.
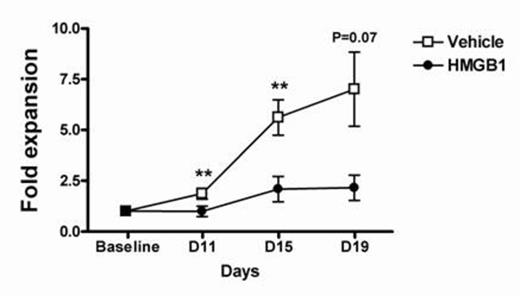
Methods and results: In order to investigate the kinetics of anemia of sepsis survivors, we first induced severe polymicrobial abdominal sepsis by surgical cecal ligation and puncture (CLP) in BALB/c mice. Sham-operated mice were used as controls. Within three days after CLP, hematocrit decreased significantly in CLP survivors three days after CLP (p≤0.001), and hemoglobin (Hb) decreased significantly within 5-7 days. Initially normocytic and normochromic, mean corpuscular hemoglobin (MCH) level was reduced significantly 15 days after CLP, while MCV dropped significantly 20 days after CLP. Sepsis-induced anemia persisted for at least 30 days. Administration of an anti-HMGB1 neutralizing monoclonal antibody (nmAb) reversed anemia. Daily administration of recombinant HMGB1 for one week was sufficient to significantly reduce Hb(p≤0.05), and MCH (p≤0.01).
To better understand the role of HMGB1 during erythropoiesis, we isolated CD34+ cells from cord blood and derived them in a methylcellulose-based culture, as well as in three phase liquid culture systems. In methylcellulose, all-thiol HMGB1 (the inflammatory form secreted during necrosis) significantly reduced BFU-E formation (P≤0.0001) in a concentration-dependent way, while sulphonyl-HMGB1 (the fully oxidized form released during apoptosis) had no effect. Moreover, all-thiol HMGB1 disrupted the architecture of BFU-E colonies. In liquid culture, HMGB1 significantly reduced CD34+ cell proliferation (Fig. 1A), and reduced the proportion of GPA+ cells by flow cytometry. In the liquid culture system, all-thiol HMGB1 reduced the proportion of GPA+, CD71+ cells by D16. Recently, CXCR4 was recognized as the receptor needed for all-thiol HMGB1 signaling, after forming a heterodimer with CXCL12. In the three-phase culture system, all-thiol HMGB1 significantly reduced the proportion of GPA+, CD71+ cells, and a similar level of inhibition was found when interfering with an anti-CXCR4 nmAb, CXCR4 by adding CXCL14 (a competitive inhibitor of CXCL12), or a small molecule (AMD3100) (Fig. 1B). In the presence of HMGB1, adding CXCL12 failed to rescue erythropoiesis.
Conclusions: Our findings suggest that all-thiol HMGB1, the redox form present during the first weeks after sepsis onset, has a critical role in initiating and sustaining anemia in sepsis survivors. Further, all-thiol HMGB1 interferes with late erythropoiesis through a CXCR4-dependent mechanism. This has potential therapeutic implications for a persistent disorder for which the usual support –including erythropoietin and transfusions- fails to improve outcome, survival, or both.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal