Abstract
Background
Large representative population-based studies in acute myeloid leukemia (AML) are scarce and restricted to reports from Sweden including cohorts until the year 2006 (Derolf, Blood 2009 & Juliusson, Blood 2009). So far, long-term data on primary treatment and trial participation in an unselected AML population are lacking. We conducted a nationwide population-based study to assess patterns of primary treatment, trial participation and survival among adult patients diagnosed with AML in the Netherlands from 1989 to 2012.
Methods
We selected all adult patients (≥18 years) diagnosed between 1989 and 2012 with acute promyelocytic leukemia (APL; N = 585; median age, 52 years; 47% males) and non-APL AML (N = 12,032; median age, 66 years; 54% males) from the nationwide population-based Netherlands Cancer Registry (NCR). Data on primary treatment for individual patients, that is, supportive care only, chemotherapy (CT) and stem cell transplantation (SCT), were retrieved from the NCR. We obtained data on the type of SCT, that is, autologous (autoSCT) or allogeneic SCT (alloSCT), from the HOVON SCT working group registry, and data on trial participation from the HOVON and EORTC cooperative trial groups. Trial participation of APL patients was outside the scope of this study. Patients were categorized into four calendar periods (1989-1994, 1995-2000, 2001-2006 and 2007-2012) and five age groups (18-40, 41-60, 61-70, 71-80 and >80 years), unless otherwise stated. We calculated relative survival rates (RSRs) as a measure of disease-specific survival.
Results
The overall age-standardized incidence of non-APL AML (3.0 per 100,000) and APL (0.15 per 100,000) remained stable over time. The proportion of patients with non-APL AML and APL diagnosed in individuals >60 years of age were 65% and 36%, respectively.
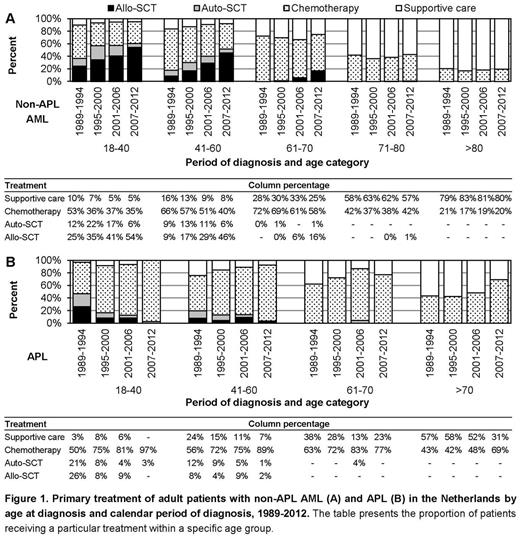
In the overall series, 40%, 52% and 64% of the patients with non-APL AML in the first three age groups received CT, 15%, 10% and <1% received autoSCT and 38%, 27% and 7% received alloSCT, respectively. The use of alloSCT for non-APL AML increased over time among patients up to 70 years of age and were gradually applied in patients 61-70 years of age since the early 2000s (Fig 1A). Among patients with non-APL AML over 70 years of age, treatment remained conservative over time (Fig 1A). Overall, 77%, 78%, 75% and 52% of patients with APL aged 18-40, 41-60, 61-70 and >70 years received CT. The use of CT for APL increased over time in all age groups (Fig 1B).
When a clinical trial was open for accrual, the inclusion rate was 68%, 57%, 30%, and 11% among patients with non-APL AML in the first four age groups, respectively. Of the patients that were not entered into a clinical trial and survived at least 30 days after diagnosis, 90%, 85%, 73% and 41% in the first four age groups received intensive therapy (CT, auto- and alloSCT) off-study, respectively.
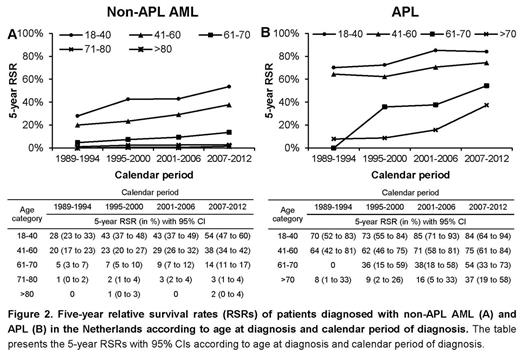
There was an overall improvement in RSRs over the study period among patients with non-APL AML and APL. The 5-year RSRs (with 95% confidence interval) increased from 12% (11%-14%), to 20% (18%-21%) for non-APL AML and from 45% (35%-54%) to 66% (58%-74%) for APL in the first and last calendar period, respectively. This improvement was mainly confined to patients with non-APL AML (Fig 2A) and APL (Fig 2B) up to 70 years of age. In addition, the RSRs also increased over time in elderly patients with APL above 70 years of age (Fig 2B).
Conclusions
In this comprehensive population-based study, we found that survival over the past two decades increased among patients with non-APL AML up to 70 years of age and among patients with APL in all age groups. This may be explained by the increased use of intensive and potentially curative treatment over time. The inclusion rate in clinical trials that employ intensive treatment approaches decreased with age, with a particular low accrual in patients above the age of 60. Survival remained poor and unchanged among patients with non-APL AML over 70 years of age, possibly due to the lack of age-adapted treatment approaches and the lack of clinical trials addressing the issue of age and frailty. Therefore, there is a need to design specific trials with innovative treatment strategies in elderly, frail patients who are not eligible for current clinical trials.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal