Abstract
Background: VRD (bortezomib, lenalidomide, dexamethasone) and Rd (lenalidomide, dexamethasone) are standard regimens for upfront treatment of multiple myeloma (MM). VRD is preferred by many centers due to higher response rates although no survival difference has been shown so far. Higher risk for side effects, especially peripheral neuropathy, higher cost, and the retrospective observation of inferior survival in genetically high risk myeloma with use of 3- compared to 2-drug regimens containing novel agents (Baz R et al. ASH 2011, abstract # 1878) puts this practice into question. Since no test predicts who may experience harm from insufficient or excessive therapy we designed a carepath that starts with 2 drugs but adapts therapy early to achieve uniform MM control.
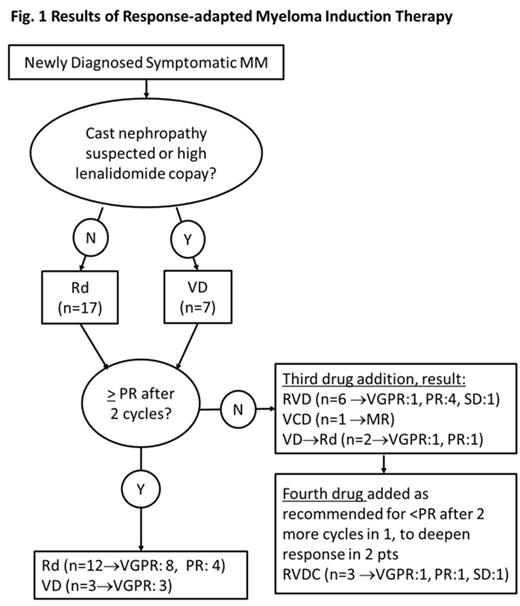
Methods: Newly diagnosed symptomatic MM patients (pts) with measurable disease who are not eligible for or elect against a clinical trial are advised to begin a 2-drug regimen of lenalidomide (R) and weekly dexamethasone (d) or, if cast nephropathy suspected or R copay too high, bortezomib (V) and dexamethasone (D). Depending on response assessment per IMWG 2011 criteria after each cycle, treatment intensity is increased with sequential addition of agents (R or V, cyclophosphamide, and then liposomal doxorubicin) when there is not at least minor response (MR) after the first, or at least partial response (PR) after the second cycle of an administered combination. Once PR is reached pts are evaluated for high dose melphalan (HDM) and ASCT or consolidation with their induction regimen followed in either case by lenalidomide maintenance. The carepath includes supportive care recommendations for use of bisphosphonates, shingles prophylaxis, and thromboembolism prophylaxis. After IRB approval, pts treated by two physicians who piloted the carepath were identified through our registry and electronic medical records were reviewed.
Results: From Oct 2012 to March 2014 the carepath was used in 24 pts. Their median age was 61 (42-82), SD stage was IIIA in 17, IIIB in 4, and IIA in 3; median ISS stage was II (III, II, and I in 9,5,10 pts, respectively), and of 18 pts with informative MyPRS® and/or FISH, 3 (17%) had high risk features (del17p, t4:14, MyPRS® score 76.04). Fifteen pts (62.5%) received 2 drugs, seven (29%) 3 drugs, and four (12.5%) were treated with 4 drugs (fig.1). Three pts underwent HDM and ASCT. Since bone marrow examinations to diagnose complete remission were not required, the best response so far is very good partial response (VGPR) in 14 pts (58%), PR in 7 pts (29%), MR in 2 pts (8%), and stable disease (SD) in 1 pt (4%) (fig.1). After median follow up of 10.7 months (5.3-20.3) one pt experienced progression after initial PR on 2 drugs and no pt has died. Bisphosphonates, shingles and DVT prophylaxis were used according to carepath in all pts. Hospital admission for a MM or treatment related complication was required in one pt (vertebral compression fracture while in PR on IV bisphosphonates); DVT (below-the-knee) on aspirin prophylaxis occurred in 2 pts (8%), and 2 pts (8%) developed peripheral neuropathy (grade 2 in one; transient grade 3 while on twice a week bortezomib in the other).
Conclusions: Starting myeloma induction with Rd or VD, with use of additional agents only in patients who do not respond early, achieved uniform disease control that required 2 drugs in about 60%, 3 drugs in about 30%, and 4 drugs in about 10% of pts in this carepath pilot. Compared to VRD for all pts, response-adapted therapy reduces neuropathy risk and cost (savings for drug alone in this cohort > $4000 / cycle). Overall survival will be the ultimate assessment of the utility of this approach, but response assessments to date support ongoing utilization of the carepath.
Off Label Use: Lenalidomide as frontline therapy for multiple myeloma. Faiman:Celgene: Speakers Bureau; Millennium / Takeda: Speakers Bureau; Onyx / Amgen: Speakers Bureau. Reu:Celgene: Research Funding; Novartis: Research Funding; Millennium / Takeda: Consultancy, Research Funding; Onyx : Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal