Abstract
Background:
With the expanding pool of cancer survivors, therapy-related AML (t-AML) is increasingly encountered and constitutes approximately 10%-20% of newly diagnosed patients with AML. These patients have a poor prognosis with conventional anti-leukemia therapies with median survival of less than 1 year. This outcome is related to several factors including older age, worse performance score, comorbidities, therapy resistance, and bone marrow failure. Allogeneic hematopoietic stem cell transplantation, when feasible, represents a potential promising treatment for patients with t-AML.
Objective:
The aim of this study is to evaluate the different treatment options and outcomes of patients with t-AML followed at our center between year 2007 and 2013.
Patients and methods:
Between July 2007 and September 2013, we evaluated 166 consecutive newly diagnosed t-AML patients among a total of 572 AML diagnosed and followed in our center during this period; there were 95 (57%) males and 71 (43%) females with a median age of 66 years (range: 18-92), 48 (29%) were less than 60 years old, 83 (50%) were between 60 and 75 years, and 35 (21%) were older than 75 years; 128 (77%) were secondary AML after hematological malignancies [66 (56%) myelodysplastic syndrome (MDS) and 44 (34%) myeloproliferative syndromes and 18 (14%) lymphoid malignancies], and 38 (23%) after solid tumors. At diagnosis, the patients were evaluated according to both cytogenetic and molecular biology data as proposed by the European LeukemiaNet (Dohner et al. Blood 2010), accordingly, 160 (96%) patients were unfavorable [having one or more of the following: -5, -7, 5q-, 7q-,11q23 or MLL+ including MLL-PTD (except 9;11), t(6;9) Complex karyotype (≥ 3 abnormalities), 3q26 abnormalities or EVI-1+], and only 6 (4%) had favorable prognosis [t(8;21), in(16) or t(16;16)]. Following the Acute Leukemia French Association (ALFA) guidelines for allo-HSCT in AML, all patients in this study should be candidate for transplantation after induction chemotherapy because of t-AML only if the patient condition is suitable (age, co-morbidities) and hematopoietic stem cell donor is available.
Results:
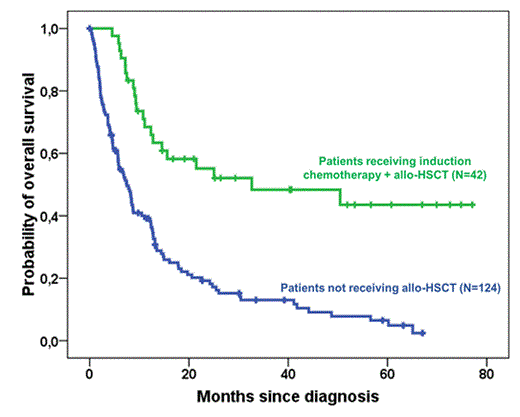
Among 166 patients, only 42 (25%) have been treated according to the ALFA recommendations and received induction chemotherapy followed by allo-HSCT, 36 (86%) were in first complete remission (CR1) and 6 were in less than CR1, 18 (43%) received hematopoietic stem cells from identical siblings (15 peripheral blood stem cells, 3 bone marrow) and 24 (57%) from unrelated donors (16 peripheral blood stem cells, 3 bone marrow and 5 cord blood cells); 31 (74%) received reduced intensity conditioning, 6 (14%) myeloablative and 5 (12%) sequential chemotherapy conditioning. For the rest of 124 (75%) patients, they could not benefit from allo-HSCT, they received either induction chemotherapy ± consolidation treatment or chemo/palliative treatment, among them 91 (73%) were ineligible to allo-HSCT due to age > 65 years and 33 (27%) could not be transplanted due to either non-availability of stem cells donor (N=12), early death before donor search (N=6), early death despite availability of stem cells donor (N=8), other comorbidities (N=7).
The probability of overall survival (OS) at 2 years was 55% (range: 47-63) for patients who received allo-HSCT and it was 18% (range: 14-22) for those who received induction or palliative treatment without allo-HSCT with a median OS of 33 and 7 months respectively. The probability of progression-free survival for patients who received allo-HSCT was 68% (range: 60-76) at two years with a median not reached, and the cumulative incidence of transplant-related mortality was 24% (range: 17-31) at two years.
Conclusion:
Therapy-related acute myeloid leukemia remains having a poor prognosis with conventional therapy. Allogeneic HCT represents the only curative approach. We obtained encouraging results in eligible patients who received allogeneic transplantation especially that the majority of patients had unfavorable prognosis factors according to molecular biology and cytogenetics.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal