Abstract
Background: Blocking lymphocyte trafficking after allogeneic stem cell transplantation (alloSCT) may prevent graft-versus-host disease (GvHD) without interfering with graft-versus-tumor (GvT) activity. We previously reported that brief (up to day+30) CCR5 blockade using maraviroc (MVC, Pfizer) after reduced-intensity conditioned (RIC) alloSCT resulted in a low incidence of acute GvHD and absence of early liver and gut GvHD, although delayed GvHD still occurred. We designed a phase II study to test the hypothesis that extended administration of MVC would be feasible, safe and provide protection against late-onset GvHD without impairing immune reconstitution or GvT responses.
Patients and Methods: In April 2013 we initiated a 37-patient (pt) phase II study to test an extended course of MVC in recipients of RIC alloSCT from unrelated donors. Pts receive fludarabine 120 mg/m2 and busulfan i.v. 6.4 mg/kg followed by peripheral blood stem cells. MVC 300 mg b.i.d. is orally administered from day -3 to day +90 in addition to standard prophylaxis with tacrolimus and methotrexate. The primary endpoint is the cumulative incidence of grade 2-4 acute GvHD by day 180. As of July 2014 we enrolled 20 pts at high risk for transplant-related toxicity by virtue of age (median=64, range 55–72), donor source (matched unrelated 80%, single-antigen mismatch unrelated 20%) or comorbidities (comorbidity index: low 15%, intermediate 35%, high 50%). Underlying diseases were AML (16), MDS, MPD, ALL and CTCL (1 each).
Feasibility and Safety: The median follow-up on surviving patients is 5.7 months. The 3-month course of MVC was well tolerated with no increased toxicity; two pts did not complete their treatment due to early disease relapse and one patient discontinued therapy due to a skin reaction with eosinophilia where the histological features favored a drug reaction and the attribution to MVC was possible. Postural hypotension, a known dose-dependent toxicity, was observed in one pt who completed the course with a 50% dose reduction.
Engraftment and Immune Reconstitution: The median time to ANC>500/μL was 12 d (range 10-21) and platelets>20k/μL was 14 d (range 9-28). The median whole blood and T-cell donor chimerism levels at day 100 were 95% (range 12–100%) and 80% (range 23–94%) respectively, which are similar to historical rates. Median CD4 counts on day 30 were 341 (range 206-424). Only 3/16 evaluable pts had Ig levels<500 mg/dL in the first 100 days.
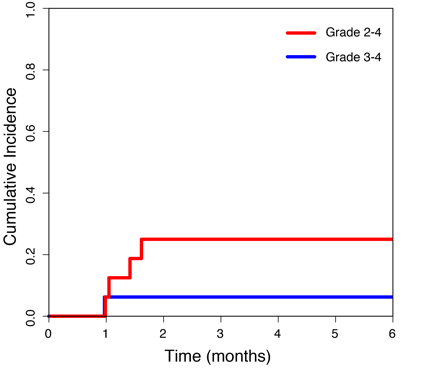
GvHD: Sixteen pts are evaluable with > 3 mo of follow-up. The day-180 cumulative incidence rates (± s.e.) of grade 2-4 and grade 3-4 acute GvHD are 25 ± 11% and 6 ± 6% respectively (Fig. 1). Of patients who developed acute GvHD in the first 180 days, there have been no cases of liver GvHD, 2 cases of stage 1 steroid-responsive gut GvHD and 1 case of severe diarrhea with combined features of GvHD and leukemic infiltrates in the gut. These results are comparable to the GvHD rates in our phase I/II MVC study (grade 2-4: 23.6% and grade 3-4: 5.9%), which included related and unrelated donor transplants. These results also compare favorably with a 45% day-180 acute GvHD rate seen in similar patients treated with our standard GvHD prophylaxis alone. Notably, there has been no treatment-related mortality. Five patients have relapsed at a median of 2.6 months post-transplant (range 0.93 – 3.5), which is similar to our historical rates after RIC alloSCT.
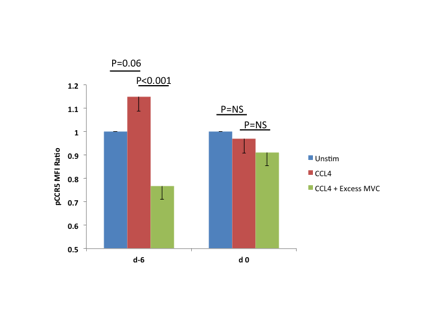
PD analysis: We developed a phosphoflow assay to assess in real-time the activity of MVC in fresh blood samples. The assay quantifies the activation of CCR5 by measuring the phosphorylation of C-terminal serine residues as a result of CCL4 stimulation. In 15 evaluable patients, we observed diminished pCCR5 levels with CCL4 stimulation on day 0 as compared to day -6 (Fig. 2).
In summary, our preliminary results support the feasibility, safety and protective activity of the CCR5 antagonist MVC against acute GvHD, with preferential activity against visceral GvHD. Continued pt enrollment and follow-up are ongoing. Updated safety, efficacy and PD results will be presented. A multi-center study (BMT-CTN 1203) will be initiated later this year to further clarify the role of this novel strategy in improving the outcome of alloHSCT.
Reshef:Pfizer: Research Funding. Off Label Use: Maraviroc for graft-versus-host disease prophylaxis.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal