Abstract
Background: Lower total CD34+ cell dose and increased HLA-mismatch are known predictors of engraftment failure and higher transplant related mortality (TRM) in cord blood (CB) recipients. To compensate for cell dose, double unit grafts (DUCBT) are commonly used in adult. However, in the majority of patients (pts), only one of the two CB units engrafts. Identification of the factors that predict which unit will engraft remains elusive, although increased recipient-donor HLA-matching and larger CD3+ cell dose have been associated with the predominating unit in a single center retrospective analysis (Ramirez et al, 2012). Historically, CB units are selected by maximizing matching at the HLA-A and -B antigen and -DRB1 allelic level. Evidence supports that matching at HLA-C appears to decrease TRM, and many clinicians incorporate HLA-C antigen matching into unit selection. It is unclear, however, if HLA-C matching predicts the engrafting unit in DUCBT. This study retrospective study evaluates whether HLA-C matching is associated with the winning CB unit.
Design: Clinical data was reviewed from all pts with a hematologic malignancy receiving a DUCBT at Moffitt Cancer Center between November 13, 2009 and August 29, 2013. Chimerism studies identified the predominating unit (> 65% single unit) between day 21 and day 28. Subsequent chimerism analyses performed at a median of day 100 confirmed unit predominance. Unit selection required intermediate resolution antigen match at HLA-A, -B, -C, and high resolution allele match at -DRB1. Units were a minimum of 4/8 matched to the patient and each other with a minimum cell dose of 1.5 x 107 total nucleated cell dose (TNC) /kg. Serology for donor specific antibodies against both units was negative.
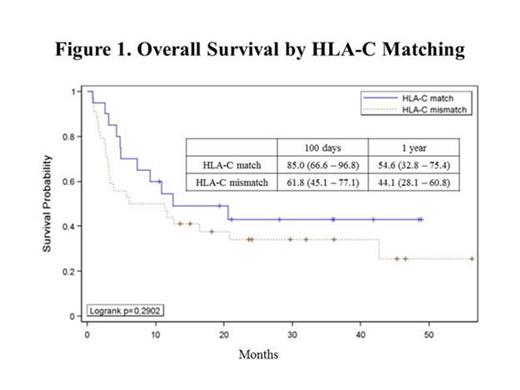
Results: Excluding 6 pts who were missing HLA-C typing on one or both CB units, 54 pts with hematologic malignancies (ALL=6, MDS/AML=29, Other=19) received chemotherapy and total body irradiation as part of a myeloablative conditioning (MAC) or reduced intensity conditioning (RIC) with or without thymoglobulin (ATG) followed by a DUCBT (MAC=14, RIC=23, RIC+ATG=17). Median age was 52 (range 22-69) years. Seven pts demonstrated persistent mixed chimerism in the myeloid and/or lymphoid cell lines beyond day 100. A total of 20 pts with available chimerism data received at least one CB unit matched to the recipient at HLA-C, with one patient excluded due to persistent mixed chimerism. Six pts received both CB units matched at HLA-C, but of the 13 pts receiving one matched and one mismatched unit, the HLA-C matched unit was the engrafting unit 69% (9/13) of the time. Comparing similar HLA mispairings, a matched unit engrafted over a mis-matched unit at HLA-A 50% (5/10) of the time, at HLA-B 38% (5/13) of the time, and at HLA-DRB1 50% (3/6) of the time. TNC dose (larger vs smaller with a required difference of at least 0.03 x 107 TNC/kg), order of infusion (first vs second unit), and overall CB unit HLA matching (4/8-8/8), were assessed as potential predictors for engraftment. Of evaluable patients, the CB unit with the larger TNC engrafted 44% (16/36) of the time, and the first unit infused was the engrafting unit 54% (21/39) of the time. In patients with an unequal overall match grade between the CB units, the better HLA-matched CB unit engrafted 64% (14/22) of the time. Survival analysis of all pts revealed that those who received at least one CB unit antigen matched at HLA-C (n=20) had a 100 day and 1 year overall survival (OS) of 85% (95% Confidence Interval(CI): 67–97%) and 55% (95% CI: 33–75), respectively, whereas pts receiving two HLA-C mismatched CB units (n=34), 100 day OS was 62% (95% CI: 45–77) with 1 year OS 44% (95% CI: 28–61) (Fig 1).
Conclusion: HLA-C antigen matching appears to help predict the winning unit in settings of HLA-match disparity and DUCBT. Confirmation in a larger population of DUCBT recipients is necessary. To date, the effects of HLA matching and other variables influencing engraftment have been predominately evaluated in recipients of single unit transplants and studies in DUCBT have been limited. Further investigation assessing HLA matching as well as donor-donor interactions is best served through a multicenter data resource.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal