Abstract
Management of acute myeloid leukemia (AML) in India remains a challenge. A major constraint is the cost of therapy. In a predominantly self paying system the majority of patients will not have the resources to manage a subsequent relapse. Hence, the choice of consolidation therapy has to be carefully considered to balance cost and efficacy. An allogeneic SCT (alloSCT) with a reduced intensity conditioning regimen (RIC) in first remission (CR1) is an attractive option to fulfill these requirements of relatively low cost without compromising efficacy. The need for consolidation chemotherapy prior to offering a RIC alloSCT for AML CR1 remains controversial. To evaluate these aspects we undertook a retrospective analysis of patients with AML CR1 who received a RIC alloSCT from multiple centers in India. Conventional criteria were used for definition of conditioning regimens to be considered RIC (CIBMTR).
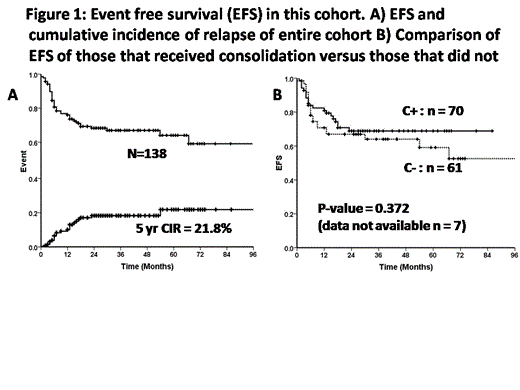
Data from 8 centers in India was collected between 2005 and 2013. A total of 138 patients fulfilled the criteria of AML CR1 having received an alloSCT with a RIC regimen. The median age was 34 years (range: 2 – 63) and 60% were males. The median time from diagnosis of AML to transplant was 99 days (range: 41 – 504). 123 (89%) were HLA matched related donors, 3 (2.1%) were MUD transplants and the rest were HLA mismatched related donors. The majority by cytogenetics (n=115) were intermediate risk (76%) followed by high risk (23%). 70 (51%) received chemotherapy consolidation prior to transplant, 61 (44%) did not and data was not available in 7 (5%). 68% of those that received consolidation received intermediate or low dose cytosine based regimens. 129 (94%) were CMV serology positive pre-transplant. Fludarabine with melphalan (140mg/m2) (128{93%}) was the most commonly used regimen and cyclosporine with short course low dose methotrexate (126{91%}) the most commonly used GVHD prophylaxis regime. All patients received a PBSC graft with a median CD34 cell dose of 9.1x106/kg (range: 1.3 – 43). With the exception of one, all patients engrafted. The median time to ANC >500/mm3 was 13 days (range: 7 – 22) and platelet count of >20,000/mm3 was 15 days (range: 0-33). Of those that engrafted, 97% achieved complete chimerism at one month post transplant (data not available in 4). Post transplant CMV reactivation was seen in 32% and a fungal infection (possible, probable or definitive) in 13%. Acute GVHD Grade 2-4 was seen in 29% and of patients evaluated 62% had chronic GVHD, the majority of these being limited (61%). The 100 day treatment related mortality (TRM) was 7.5% and the one year TRM was 25.6%. At a median follow up of 24 months the 5 year EFS and OS was 64.0±5.07 (Figure 1A) and 71.1±4.0 respectively. The 5 year cumulative incidence of relapse was 21.8% (Figure 1A). The baseline characteristics as mentioned above were not significantly different between the group that received consolidation and the group that did not. The use of consolidation therapy prior to alloSCT did not have a significant impact on EFS or OS (Figure 1B).
On univariate analysis the factors that adversely impacted EFS were mismatched non sibling family donor (RR 8.1; P-value 0.001), CMV reactivation (RR 2.6; P-value 0.001), fungal infection post transplant (RR 6.8; P-value 0.000) and acute GVHD (RR 2.1; P-value 0.02). On a forward stepwise multivariate analysis adjusting for these and other conventional risk factors only CMV reactivation (RR 2.0; 95% CI 1.03-3.87; P-value 0.042) and fungal infection (RR 7.1; 95%CI 3.154-16.12; P-value 0.000) retained their adverse impact. There was no correlation between CMV reactivation and relapse of disease post transplant.
The mean costs of induction chemotherapy for these patients was US$ 9239±3596 (n=74), for consolidation chemotherapy it was 5007±3490 (n=21) and for alloSCT it was 18138±13826 (n=118; costing up to 1 year post transplant). Induction chemotherapy followed by HLA matched RIC alloSCT is likely to be a cost effective and affordable treatment option for young adults with AML in CR1in an Indian context. With an average gross net income in India of US$3500/year (http://indiabudget.nic.in) the limitation still remains the cost of treatment and number of centers that can offer this therapy.
Srivastava:Octapharma: Consultancy, Other. Off Label Use: Bortezomib in the treatment of acute promyelocytic leukemia.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal