Abstract

Background: Autologous hematopoietic progenitor cells (auto-HC) can be mobilized either by hematopoietic growth factors (GF) alone or cytotoxic chemotherapy + GF (CC+GF). The latter is associated with higher CD34+ yields, but is associated with increased costs and risks of infection and hospitalization. In patients with multiple myeloma (MM) undergoing Auto-HCT, it is uncertain whether mobilization with CC+GF affects post transplant outcomes.
Methods: We conducted a retrospective analysis of patients with MM undergoing their first AHPCT following high dose melphalan (≥140 mg/m2) between 2007 and 2012 in US and Canada and registered with the Center for International Blood and Marrow Transplant Research (CIBMTR). Patients who had a planned subsequent allogeneic transplant, received VAD or similar induction therapy, had disease progression prior to transplant, or were mobilized with plerixafor were excluded from the analysis. The primary endpoint was progression-free survival (PFS) and the main secondary end-points were transplant-related mortality (TRM), relapse and overall survival (OS).
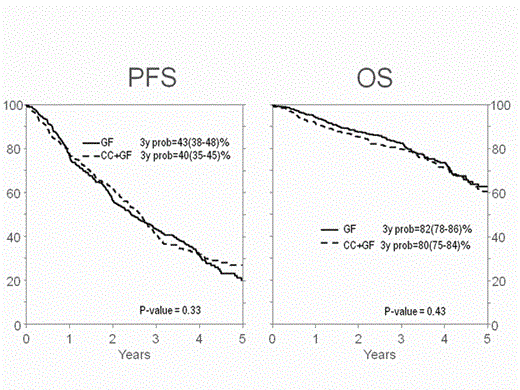
Results: There were 519 patients in GF and 449 in CC+GF with median follow-up of 42 and 46 months respectively. The most common mobilizing CC regimen was single agent cyclophosphamide (71%). GF and CC+GF groups were similar but there were more patients in GF with only one prior line of therapy, lenalidomide exposure and CR at the time of transplant and more patients in CC+GF with low HCT co-morbidity index, prior thalidomide exposure and planed second autologous transplant (Table 1). Median number of days for auto-HC collection was 2 (IQR 1-3) in GF and 1 (IQR 1-3) in CC+GF (P<0.001). There were fewer days between collection and transplant (median 16 vs. 18, P<0.001) and fewer CD34+ cells (x 106/kg) infused at transplant (median 3.9, IQR=3.1-4.8, vs. 5.1, IQR 3.5-6.9, P<0.001) in GF than in CC+GF. Kinetics of neutrophil engraftment (> 0.5 x 109/L) was similar between groups (13 vs. 13 days, P=0.69) while platelet engraftment (> 20 x 109/L) was faster in CC+GF (median 19 vs. 18 days, P=0.006). There was no difference between groups in number of hospitalization days (14 vs. 14, P=0.7). In univariate analysis, there was no significant difference between the two groups in OS, PFS, or TRM. In multivariate analysis, stage III at diagnosis and Karnofsky status <90 but not modality of mobilization were associated with worse PFS. Similarly HCT-CI >2, Stage III at diagnosis and immunoglobulin isotype (IgG/IgA/Others), but not mobilization were associated with OS. Adjusted 3-years PFS was 43% (95% C.I. 38-48) in GF and 40% (95% C.I. 35-45) in CC+GF, P=0.33 (Figure). Adjusted 3-years OS was 82% (95% C.I. 78-86) vs 80% (95% C.I. 75-84), P=0.43 and adjusted 5-year OS was 62% (95C.I. 54-68) vs. 60% (95% C.I. 52-67), P=0.76, for GF and CC+GF respectively (Figure).
Conclusions: MM patients undergoing AHPCT have similar outcomes irrespective of the use of cytotoxic chemotherapy mobilization. We found no evidence that chemotherapy mobilization contributes to disease control in MM.
Characteristics of patients and treatments
| GF | CC+GF | P | |
| N=519 | N=449 | ||
| Lines of therapy | <0.001 | ||
| 1 | 382 (74) | 265 (59) | |
| 2 | 113 (22) | 136 (30) | |
| >2 | 24 (5) | 48 (11) | |
| Prior therapy | <0.001 | ||
| Thalidomide+Bortezomb+-Steroid | 74 (14) | 92 (20) | |
| Lenalidomide+Bortezomb+-Steroid | 121 (23) | 48 (11) | |
| Thaildomide+-Steroid | 85 (16) | 106 (24) | |
| Bortezomib+-Steroid | 132 (25) | 136 (30) | |
| Lenalidomide+-Steroid | 107 (21) | 67 (15) | |
| Disease status at HCT | 0.05 | ||
| CR | 84 (16) | 48 (11) | |
| PR | 407 (78) | 378 (84) | |
| MR/NR/SD | 28 (5) | 23 (4) | |
| HCT-CI | 0.006 | ||
| 0 | 227 (44) | 227 (51) | |
| 1-2 | 147 (29) | 134 (30) | |
| >2 | 145 (28) | 88(20) | |
| Time from diagnosis to HCT | <0.001 | ||
| <6 mo | 221 (43) | 140 (31) | |
| 6-12 mo | 298 (57) | 309 (69) | |
| Year of HCT | 0.03 | ||
| 2007-2008 | 289 (56) | 272 (61) | |
| 2009-2010 | 105 (20) | 100 (22) | |
| 2011-2012 | 125 (24) | 77 (17) | |
| Melphalan dose | 0.40 | ||
| 140-180 mg/m2 | 71 (14) | 70 (16) | |
| >180 mg/m2 | 448 (86) | 379 (84) | |
| Transplant type | 0.02 | ||
| Single transplant | 417 (80) | 331 (74) | |
| Multiple Transplants | |||
| Planned 2ndAuto (0-6 mo) | 42 (8) | 63 (14) | |
| Planned 2ndAuto (>6 mo) | 5 (1) | 6 (1) | |
| Use of maintenance agent | 198 (38) | 176 (39) | 0.29 |
| GF | CC+GF | P | |
| N=519 | N=449 | ||
| Lines of therapy | <0.001 | ||
| 1 | 382 (74) | 265 (59) | |
| 2 | 113 (22) | 136 (30) | |
| >2 | 24 (5) | 48 (11) | |
| Prior therapy | <0.001 | ||
| Thalidomide+Bortezomb+-Steroid | 74 (14) | 92 (20) | |
| Lenalidomide+Bortezomb+-Steroid | 121 (23) | 48 (11) | |
| Thaildomide+-Steroid | 85 (16) | 106 (24) | |
| Bortezomib+-Steroid | 132 (25) | 136 (30) | |
| Lenalidomide+-Steroid | 107 (21) | 67 (15) | |
| Disease status at HCT | 0.05 | ||
| CR | 84 (16) | 48 (11) | |
| PR | 407 (78) | 378 (84) | |
| MR/NR/SD | 28 (5) | 23 (4) | |
| HCT-CI | 0.006 | ||
| 0 | 227 (44) | 227 (51) | |
| 1-2 | 147 (29) | 134 (30) | |
| >2 | 145 (28) | 88(20) | |
| Time from diagnosis to HCT | <0.001 | ||
| <6 mo | 221 (43) | 140 (31) | |
| 6-12 mo | 298 (57) | 309 (69) | |
| Year of HCT | 0.03 | ||
| 2007-2008 | 289 (56) | 272 (61) | |
| 2009-2010 | 105 (20) | 100 (22) | |
| 2011-2012 | 125 (24) | 77 (17) | |
| Melphalan dose | 0.40 | ||
| 140-180 mg/m2 | 71 (14) | 70 (16) | |
| >180 mg/m2 | 448 (86) | 379 (84) | |
| Transplant type | 0.02 | ||
| Single transplant | 417 (80) | 331 (74) | |
| Multiple Transplants | |||
| Planned 2ndAuto (0-6 mo) | 42 (8) | 63 (14) | |
| Planned 2ndAuto (>6 mo) | 5 (1) | 6 (1) | |
| Use of maintenance agent | 198 (38) | 176 (39) | 0.29 |
Costa:Sanofi: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal