Abstract
Ribonuclease Inhibitor (RNH1) is a ubiquitously expressed leucine-rich repeat protein. The human RNH1 gene evolved via gene duplication and is conserved among mammalian species. RNH1 binds to and inhibits pancreatic type ribonucleases. Further, RNH1 contains numerous cysteine residues whose sulfhydryl groups might play key structural roles and protect from oxidative damage (Dickson et al Prog. Nucleic Acid Res. Mol. Biol 2005). Despite of all these observations, the precise biological role of RNH1in vivo remains unexplored. Here, we describe an essential role for Rnh1 in the regulation of erythropoiesis by controlling erythroid differentiation.
To understand the biological function of Rnh1, Rnh1-deficient (Rnh1-/-) mice were generated. Rnh1-/- embryos die between embryonic days E8.5 to E10 due to severe decrease in erythroid cells. Similar percentages of c-Kit+CD41+ cells (Hematopoietic stem/progenitor cells) were present in Rnh1-/- yolk sacs compared to control genotypes, however differentiation of mature erythroid cells was impaired. Rnh1 is expressed in erythroid cells and its expression coincides with the site of primitive erythropoiesis in the yolk sac. Gene expression studies revealed that levels of hematopoietic transcription factors (TF) in Rnh1-deficient yolk sacs were normal, but their target genes were down-regulated. These results indicate that a post-transcriptional mechanism that affects TF gene function. Supporting this, protein levels of the erythroid transcription factor GATA1 and PPARγ, previously shown to control the proliferation and differentiation of erythroid progenitors, were selectively impaired. Whereas myeloid transcription factors C/EBPa and C/EBPb were not affected in Rnh1-/- embryos, suggesting that Rnh1 deficiency specifically affects the translation of erythroid transcription factors.
At the molecular level, using the human erythroid K562 cell line, we show that RNH1 is recruited to the ribosome complex and binds to the ribosomal proteins. RNH1-deficiency decreased polysome formation and conversely its overexpression increased polysome formation. Increased expression of RNH1 also increased globin gene expression in K562 cells. These results suggest that RNH1 associates with ribosomes and regulates the translation of erythroid-specific genes, which are necessary for erythroid differentiation. Furthermore, Rnh1 haploinsufficiency leads to decreased erythropoiesis in the spleen of adult mice.
Ribosomal haploinsufficiency in several ribosomal genes is known to impair ribosome function and cause macrocytic anemia in Diamond–Blackfan anemia (DBA), a congenital bone marrow failure syndrome, and the 5q- syndrome, a subtype of myelodysplastic syndrome (Narla et al Int. J. Hematol 2011). Recently it has been shown that ribosomal haploinsufficiency can specifically cause a decrease in GATA1 mRNA translation (Ludwig et al Nature Med 2014). Similar to these ribosomal genes, we demonstrate that Rnh1 associates with ribosomes and its deficiency impairs the translation of Gata1 and other erythroid-specific transcription factors, which leads to arrest in erythroid maturation. Collectively our results unravel the important biological function of Rnh1 in the regulation of erythropoiesis, and point to novel therapeutic targets for disorders of erythropoiesis involving ribosomal defects.
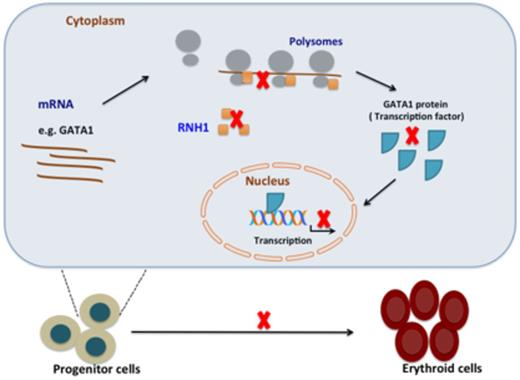
RNH1 is recruited to ribosomal complex and is involved in translation of erythroid specific transcription factors (TF) e.g.GATA1. These TFs are necessary for differentiation of progenitor cells in to erythroid cells. RNH1 deficiency impairs the translation of GATA1 and other erythroid-specific transcription factors, which leads to arrest in erythroid maturation.
RNH1 is recruited to ribosomal complex and is involved in translation of erythroid specific transcription factors (TF) e.g.GATA1. These TFs are necessary for differentiation of progenitor cells in to erythroid cells. RNH1 deficiency impairs the translation of GATA1 and other erythroid-specific transcription factors, which leads to arrest in erythroid maturation.
Summary Figure:RNH1 is recruited to ribosomal complex and is involved in translation of erythroid specific transcription factors (TF) e.g.GATA1. These TFs are necessary for differentiation of progenitor cells in to erythroid cells. RNH1 deficiency impairs the translation of GATA1 and other erythroid-specific transcription factors, which leads to arrest in erythroid maturation.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal