Abstract
Background. Minimal residual disease (MRD) is powerful tool for prediction of treatment outcome in leukemia patients of various age groups, including infants with acute lymphoblastic leukemia (ALL). In the vast majority of cases only bone marrow (BM) samples are used for MRD detection.
Objective. To estimate prognostic significance of MRD in peripheral blood (PB) and BM by qualitative detection of different MLL fusion gene transcripts in infant ALL enrolled into MLL-Baby protocol.
Methods. Fifty three infants (20 boys and 33 girls) with median age of 5.3 months (range 0.03-11.80) and defined MLL rearrangements were included in the current study. Among them there were 25 patients (47.2%) carrying MLL-AFF1 fusion gene transcripts, 10 (18.9%) MLL-MLLT3-positive cases, 9 (17.0%) MLL-MLLT1-positive cases, 5 (9.4%) MLL-MLLT10-positive cases and 4 (7.5%) MLL-EPS15-positive ones. MRD evaluation was performed by detection of MLL fusion gene transcripts in BM and PB samples using real-time PCR and nested RT-PCR with sensitivity non-less than 1E-04. MRD-negativity was defined as absence of fusion gene transcripts in both assays. Median of follow-up period in the observed group was 5.2 years. Time points (TP) for MRD assessment were as follows: day 15 of remission induction (TP1), at the end of remission induction (TP2), after each course of ATRA administration (TP3-TP7). Informed consent was obtained in all cases.
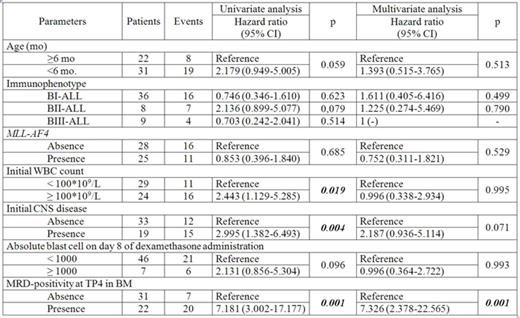
Results. We estimated 142 paired BM/PB samples. 77 samples were double positive, 43 were double negative Thus concordance between MRD results in BM and PB samples achieved 84.5%. Concordance varied between different TPs of MLL-Baby protocol from 79.0% to 100%. The highest concordance rate was at TP4 and TP7 (92.3% and 100%, respectively). Interestingly, all discrepant results (22 samples 15.5%) were BM-positive/PB-negative. Median level of ABL gene, used for normalization, was similar in BM and PB samples (4.85E+04 vs 4.95E+04, respectively, p=0.760). Evaluation of prognostic significance of MRD in BM in TP1-TP7 revealed that TP4 was the earliest TP when discriminative data between MRD-positive and MRD-negative patients were obtained. MRD-positivity at TP4 in BM led to unfavorable outcome. Event-free survival was significantly lower in MRD-positive group (n=22) in comparison to MRD-negative one (n=31) (0.06±0.06 vs 0.70±0.09 p=0.0001), while cumulative incidence of relapse in MRD-positive patients was remarkably higher (0.92±0.01 vs 0.29±0.08, p<0.0001). MRD-positivity at this TP in BM was the only significant factor in the diagnostic model where initial risk factors (age at diagnosis, initial WBC count, immunophenotype, CNS disease, presence of MLL-AF4) were combined to response criteria (number of blast cells at day 8 of dexamethasone prophase and MRD in BM at TP4) (Table). The only TP when MRD data obtained from PB samples had prognostic value was TP6. In this TP cumulative incidence of relapse in MRD-positive patients was significantly higher in comparison to MRD-negative ones (0.88±0.11 vs 0.25±0.13, respectively, p=0.003). However these data did not bring any extra advantages as compared to TP4 in BM.
Conclusions. Despite high qualitative concordance rate between MRD detection in BM and PB samples we could not show prognostic value of MRD monitoring in PB by fusion gene transcripts. Univariate and multivariate analysis revealed that MRD-positivity at TP4 in BM was the only significant and independent prognostic factor of unfavorable outcome in the observed group of patients.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal