Abstract
Background: Minimal residual disease (MRD) assessment at the end of induction is critical for risk stratification of B-lineage acute lymphoblastic leukemia (B-ALL) patients. There are multiple techniques for molecular MRD assessment, including next-generation sequencing (NGS) and allele-specific oligonucleotide PCR (ASO-PCR) amplification of immunoglobulin (Ig) or T-cell receptor rearrangements. Leukemia-specific rearrangements can be identified in >97% of B-ALL patients using the NGS-based LymphoSIGHT™ method, while ASO-PCR can typically identify leukemia-specific rearrangements in only 80-90% of B-ALL patients. In this report, we assessed the ability of the NGS method to predict event-free survival (EFS) in a cohort of B-ALL patients who were inevaluable by ASO-PCR.
Methods: All patients were treated between 2005-2011 on a clinical trial conducted by the Dana-Farber Cancer Institute (DFCI) ALL Consortium (05-001) on which high levels of end-induction MRD (≥10-3) led to intensified treatment. The 37 pediatric B-ALL patients included in this study were inevaluable by ASO-PCR, and therefore this cohort represents a subset of patients in whom a decision to intensify therapy per protocol could not be made. Archived DNA samples from diagnosis and Day 32 were provided for MRD assessment using the NGS method. Using universal primer sets, we amplified the immunoglobulin (IGH-VDJ, IGH-DJ and IGK) and T cell receptor (TRB, TRD, TRG) loci in diagnostic bone marrow samples. Leukemia-specific clonotypes were identified in the diagnostic sample of each patient based on high-frequency within the B-cell and/or T-cell repertoire. The presence of leukemia-specific clonotypes was then assessed in the end-induction (Day 32) sample. NGS MRD assessment was performed blinded to all clinical and ASO-PCR information on these patients. The association of NGS MRD results and clinical outcome (EFS) was examined, and significance assessed using the log-rank test.
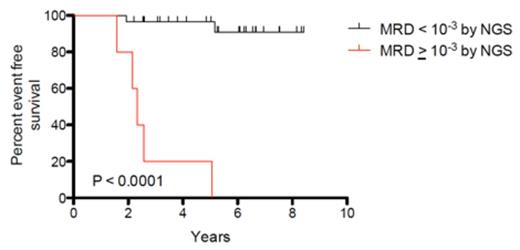
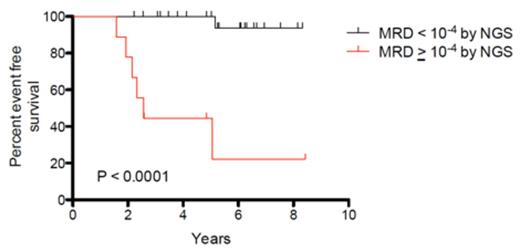
Results: Of 37 patients who were inevaluable for end-induction MRD by ASO-PCR, leukemia-specific clonotypes were successfully identified in 35 (95%) by the NGS method. The use of a threshold of 10-3, which is the ASO-PCR threshold used for risk-stratification on DFCI protocols, demonstrated significantly improved EFS between the MRD negative and MRD positive groups (p<0.0001) (Figure 1A). A similar analysis with a threshold of 10-4 also demonstrated significantly improved EFS between the MRD negative and MRD positive groups (p<0.0001) (Figure 1B).
Conclusions: This study demonstrates the prognostic significance of NGS-based MRD assessment in pediatric patients with B-ALL. This NGS-based assay led to successful assessment of MRD in 95% of patients who were inevaluable by ASO-PCR. The NGS method represents an alternative approach for clinical MRD monitoring that could fundamentally improve the ability to identify high-risk B-ALL patients and potentially improve their outcome.
Kaplan-Meier analysis of event-free survival for A) 30 MRD negative (<10-3) and 5 MRD positive (≥10-3) patients and B) 26 MRD negative (<10-4) and 9 MRD positive (≥10-4) patients.
Kaplan-Meier analysis of event-free survival for A) 30 MRD negative (<10-3) and 5 MRD positive (≥10-3) patients and B) 26 MRD negative (<10-4) and 9 MRD positive (≥10-4) patients.
Zheng:Sequenta, Inc.: Employment, Equity Ownership. Faham:Sequenta, Inc.: Employment, Equity Ownership, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal