Abstract
Introduction: Thrombotic thrombocytopenic purpura (TTP) is a hematologic emergency that requires the immediate initiation of therapy without access to the results of definitive testing and often in the setting of limited prior clinical experience. In this context, a simple, validated, and rapidly deployable diagnostic scoring system could be a powerful tool.
Methods: We studied 214 consecutive adults admitted to 3 major teaching hospitals between 2004 and 2014 with thrombocytopenia (range: 5 to 149,000/μL) and schistocytes suspicious for TTP (derivation cohort). Twenty-nine different laboratory and clinical parameters were evaluated to identify those most highly associated with an ADAMTS13 activity level of ≤10%. Cutoffs for continuous variables were determined by generating receiver operator curves and identifying the cutoff point associated with the maximum Youden Index. Those parameters most associated with a low ADAMTS13 level by univariate analysis (P≤0.1) were included in a logistic regression to create a parsimonious predictive model. The resulting model was validated through a prospectively collected internal cohort (n=40) and an external cohort (n=147) drawn from a different academic medical center.
Results: Four individual laboratory parameters (platelet count <30,000/μL, MCV <90fL, creatinine <2.0mg/dL, and INR <1.5), a combined laboratory parameter (evidence for hemolysis based on either a reticulocyte count >2.5%, an indirect bilirubin >2.0 mg/dL, or absent haptoglobin), and two historical features (no active cancer, no history of bone marrow or solid organ transplantation) were predictive of an ADAMTS13 activity of ≤10% in our logistic regression model. Based on similar odds ratio estimates, each factor was assigned a value of 1 point, with scores of 0-4 considered low risk, 5-6 intermediate, and 7 high. The derivation cohort (n=214) contained 62 cases of idiopathic TTP with low ADAMTS13 activity level. When applied against this cohort, the score effectively sorted patients according to ADAMTS13 level (See Table) and demonstrated an AUC of 0.96 (95% CI 0.92-0.98).
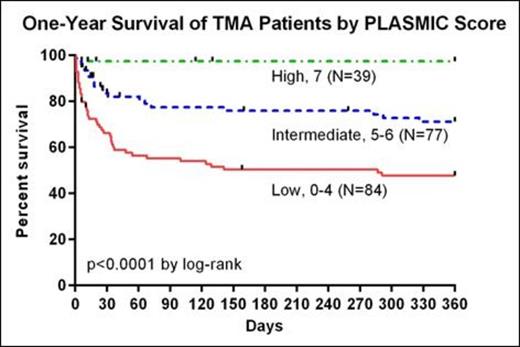
An internally-collected prospective validation cohort (n=40) contained 6 cases of idiopathic TTP with low ADAMTS13 activity, and the score demonstrated an AUC of 0.96 (95% CI 0.84-1.00). For the second independent validation, an external cohort from a large academic healthcare system in the southeastern United States was used (n=147) that contained 69 cases of idiopathic TTP with low ADAMTS13 activity. When tested against this dataset, cases with scores falling in the low, intermediate, and high risk groups were respectively 4.3%, 56.8%, and 96.2% likely to have an ADAMTS13 activity level ≤10% (see Table). This corresponded to an AUC of 0.90 (95% CI 0.84-0.95). Scores corresponded to certain diagnoses, with 21/27 cases of DIC and 23/31 cases of drug-associated thrombotic microangiopathy (TMA) sorting to the low risk category. Scores were associated with one year survivals of 46.9%, 69.2%, and 96.9% for the low, intermediate, and high risk groups respectively (see Figure, p < 0.0001 by log-rank).
Conclusions: A simple, validated clinical scoring system can assist in the early diagnosis of TTP by accurately identifying cases with a high likelihood of having severe ADAMTS13 deficiency. The word PLASMIC can be used to recall the 7 features of the score (platelets, lysis, active cancer, stem cell or solid organ transplant, MCV, INR, and creatinine). This scoring system also has the potential to provide accurate diagnostic and prognostic information for other TMAs. The ability to rapidly diagnose TTP with this approach would facilitate the initiation of appropriate therapy, while in low-risk cases clinicians could pursue alternative diagnoses with a high degree confidence.
| . | Proportion ADAMTS13 ≤10% . | ||
|---|---|---|---|
| . | Derivation (%) . | Validation . | |
| . | . | Internal (%) . | External (%) . |
| Risk Category | |||
| Low (0-4) | 0/84 (0) | 0/22 (0) | 2/47 (4.3) |
| Intermediate (5-6) | 25/77 (32.4) | 3/15 (20) | 42/74 (56.8) |
| High (7) | 37/39 (94.9) | 3/3 (100) | 25/26 (96.2) |
| . | Proportion ADAMTS13 ≤10% . | ||
|---|---|---|---|
| . | Derivation (%) . | Validation . | |
| . | . | Internal (%) . | External (%) . |
| Risk Category | |||
| Low (0-4) | 0/84 (0) | 0/22 (0) | 2/47 (4.3) |
| Intermediate (5-6) | 25/77 (32.4) | 3/15 (20) | 42/74 (56.8) |
| High (7) | 37/39 (94.9) | 3/3 (100) | 25/26 (96.2) |
Makar:Alexion Pharmaceutials: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal