Abstract
The increased survival of children with cancer is largely credited to treatment on clinical trials. As such there is interest in determining factors associated with trial enrollment. Adult oncology studies suggest non-Whites are less likely to enroll than Whites on clinical trials. Additional factors, such as insurance and geographic region, have been associated with adolescent and adult trial enrollment. Data describing factors associated with pediatric trial enrollment is limited. This study sought to evaluate whether race and other patient factors were associated with enrollment on the recently completed Children's Oncology Group (COG) acute myeloid leukemia (AML) chemotherapy trial AAML0531.
A retrospective cohort study was conducted using data from the Pediatric Health Information System (PHIS) and trial data from COG AAML0531. PHIS is an administrative database containing inpatient data from Child Health Corporation of America-affiliated hospitals. Data from a previously assembled and validated PHIS AML cohort was merged with data from the AAML0531 trial for the period of time that the COG trial was open and PHIS data was available (2006 to 2010). Patients that were identified in both the PHIS and COG datasets were labeled as enrolled patients and those only in the PHIS database were labeled as not enrolled patients. Enrolled and not enrolled patients were compared by race, gender, age, insurance type, illness severity at AML presentation (defined by need for an ICU intervention in the first or second day of index admission) and geographic region of hospitalization. Chi-square test and logistic regression analyses were used for unadjusted and adjusted comparisons, respectively.
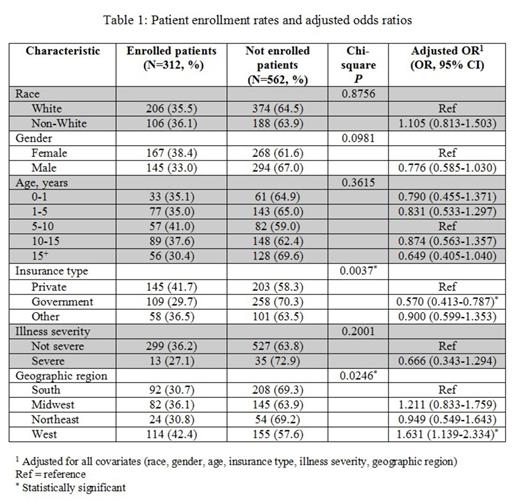
The PHIS AML cohort contained 874 patients of which 312 (36%) were enrolled on AAML0531. Table 1 displays the comparison of enrolled versus not enrolled patients by each of the demographic and clinical variables of interest. In adjusted analyses patients were less likely to enroll if they had government insurance (compared to private insurance). Patients were more likely to enroll if they were hospitalized in the West (compared to the South).
Trial enrollment percentage was higher than reported in adult cooperative group trials but still comprised a minority of potentially eligible patients. Unlike adult clinical trials, race was not associated with enrollment on the AAML0531 trial. However, patients who did enroll on AAML0531 were less likely to have government insurance and more likely to be hospitalized in the West. Work is ongoing to refine estimates of enrollment eligible patients, to define patient socio-economic status more precisely than insurance status, and to evaluate the impact of insurance status beyond socio-economic status. Such analyses should provide additional data to guide efforts to increase trial enrollment and ensure equitable access to COG AML clinical trials for all pediatric patients.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal