Abstract
Background
Approximately 10% of cases of acute myeloid leukemia (AML) arise following exposure to chemotherapy (treatment-related AML [tAML]). Compared to de novo AML patients, tAML patients have a poor prognosis, not least due to a higher proportion having adverse cytogenetics. Treatment options for tAML patients are limited and outcomes remain dismal. Median overall survival (OS) of 6.9 mo as of initial presentation, with 25% deaths within one month of diagnosis, was reported for a large cohort of tAML patients, with individualized treatments ranging from supportive care, to intensive chemotherapy (IC) and bone marrow (BM) transplantation.1 Few studies have assessed azacitidine (AZA) in patients with tAML, and those that have, did not report separate outcomes for this subgroup.2–4
Methods
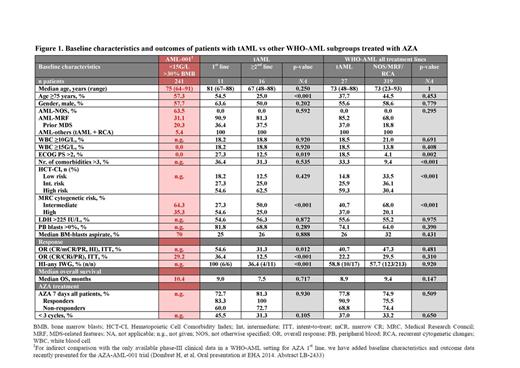
Here, we compare a subgroup of tAML patients (n=27) with patients with other World Health Organization (WHO)-AML subgroups (n=319) from the Austrian AZA Registry. The aim of the registry was to gain insight of the use, safety and efficacy of AZA in ‘real-world’ AML patients. The sole inclusion criteria were the diagnosis of WHO-AML and treatment with ≥1 dose of AZA.
Results
Baseline characteristics were compared between patients with tAML and WHO-AML: median age, gender, percentage with myelodysplatic syndrome (MDS)-related features, serum lactate dehydrogenase (LDH) level, and median peripheral blood and BM blasts were similar between both cohorts. However, tAML patients had poorer Eastern Cooperative Oncology Group Performance Status (ECOG PS) scores, more comorbidities, and a higher proportion of high-risk cytogenetics compared with WHO-AML patients.
Of patients with tAML, 41% received AZA as 1st line therapy and 59% received AZA as ≥2nd line therapy. Baseline characteristics of these two subgroups were also comparable, except that tAML patients treated with AZA 1st line were older, had poorer ECOG PS and worse cytogenetics than ≥2nd line patients, which is in line with what would be expected from a cohort not deemed eligible for IC (Figure 1).
Median number of AZA cycles was 4 (range 1–25) for tAML patients and 4 (range 1–46) for WHO-AML patients. Median time from AZA stop to death was 1.9 vs 1.8 mo for patients with tAML vs other WHO-AML subgroups.
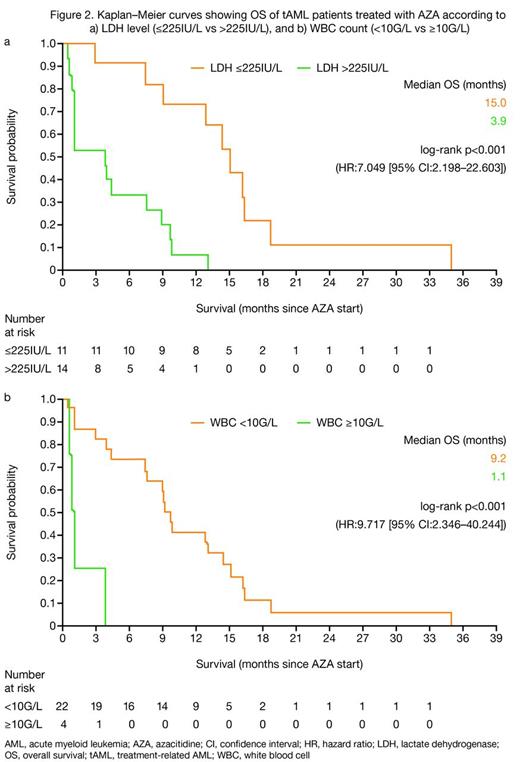
The overall response rate (ORR; International Working Group [IWG] 2003 criteria5) as well as the rate of hematologic improvement (HI; IWG 2006 criteria6) were similar for patients with tAML vs WHO-AML treated with AZA (complete response [CR] + CR with incomplete blood count recovery [CRi] + partial response [PR]: 22.2 vs 29.5%; and HI: 58.8 vs 57.7%, respectively; Figure 1). Median OS was similar for patients with tAML vs WHO-AML (8.9 vs 9.4 mo; p=0.147; Figure 1), but was longer for responders than non-responders in both groups of patients. Relapse-free survival was 7.7 vs 9.1 mo for patients with tAML vs WHO-AML. In univariate analyses, baseline factors that significantly affected OS for tAML patients were LDH >225IU/L (p<0.001) and white blood cell count ≥10G/L (p<0.001; Figures 2a, b).
Patients with tAML who received AZA 1st line achieved a significantly higher ORR than those who received AZA ≥2nd line (36.4 vs 12.5%; p<0.001). Median time to AZA start as of initial diagnosis was 0.7 and 5.1 mo for tAML patients treated with AZA 1st line or ≥2nd line, respectively. Median number of AZA cycles was 6 (1–25) for AZA 1st line vs 4 (1–15) for AZA ≥2nd line. Median OS was not significantly different for tAML patients treated with AZA 1st vs ≥2nd line (9.0 vs 7.5 mo; p=0.717; Figure 1). Median relapse-free survival was 7.7 and 7.4 mo for AZA 1st line and ≥2nd line, respectively. tAML patients achieving CR/CRi/PR had a median OS of 16.1 mo with AZA 1st line and 12.8 mo for AZA ≥2nd line.
Conclusions
This represents the largest study reporting outcomes of tAML patients treated with AZA. Survival of tAML patients with AZA was comparable to that achieved in other WHO-AML subgroups, suggesting that AZA is a feasible treatment option for this poor prognostic subgroup. The OS data compare favorably to OS data reported for IC1 and are encouraging. Based on these ‘real-world’ data, randomized trials should assess AZA in tAML patients.
1. Smith SM, et al. Blood 2003;102:43–52
2. Bally C, et al. Leuk Res 2013;37:637–40
3. Fianchi L, et al. J Hematol Oncol 2012;5:44
4. Pleyer L, et al. Ann Hematol 2014; epub ahead of print
5. Cheson BD, et al. J Clin Oncol 2003;21:4642–9
6. Cheson BD, et al. Blood 2006;108:419–25
Pleyer:Bristol-Myers Squibb: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; AOP Orphan Pharmaceuticals: Honoraria; Celgene: Consultancy, Honoraria. Off Label Use: Vidaza (azacitidine) is indicated for the treatment of adult AML patients who are not eligible for haematopoietic stem cell transplantation with 20–30 % blasts and multi-lineage dysplasia, according to WHO classification. This cohort also includes AML-patients with >30% bone marrow blasts.. Burgstaller:AOP Orphan Pharmaceuticals: Honoraria; Novartis: Honoraria; Mundipharma: Honoraria; Celgene: Consultancy. Stauder:Celgene: Consultancy, Honoraria, Research Funding; Ratiopharm: Honoraria, Research Funding; Novartis: Research Funding. Girschikofsky:Pfizer: Honoraria, Research Funding; Mundipharm: Consultancy, Honoraria. Pfeilstöcker:Janssen-Cilag: Honoraria; Novartis: Consultancy, Honoraria; Celgene: Consultancy, Honoraria. Lang:Celgene: Consultancy. Sperr:Phadia: Research Funding; Novartis: Honoraria; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees. Valent:Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees; BMS: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees. Greil:Sanofi Aventis: Honoraria; Roche: Honoraria; Pfizer: Honoraria, Research Funding; Boehringer-Ingelheim: Honoraria; Astra-Zeneca: Honoraria; Novartis: Honoraria; Genentech: Honoraria, Research Funding; Janssen-Cilag: Honoraria; Merck: Honoraria; Mundipharma: Honoraria, Research Funding; Eisai: Honoraria; Amgen: Honoraria, Research Funding; Celgene: Consultancy, Research Funding; Cephalon: Consultancy, Honoraria, Research Funding; Bristol-Myers-Squibb: Consultancy, Honoraria; GSK: Research Funding; Ratiopharm: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal