Abstract
Introduction: There have been minimal therapeutic advancements in the management of acute myeloid leukemia (AML) over the past 3 decades. Although approximately 70% of younger AML patients will achieve a complete remission (CR) with standard induction chemotherapy regimens, overall outcomes are dismal. It is unclear why drug development has been particularly hindered in AML when compared to other hematologic malignancies and cancers.
Methods: A retrospective analysis was performed on 65 phase 1 clinical trials supported by the Cancer Therapy Evaluation Program (CTEP) of the National Cancer Institute (NCI) from 1984-2009. A total of 711 adult AML patients were enrolled in these studies. The primary objectives were to assess overall response rates ([ORR]: CR + partial remission) and treatment-related mortality (all causes of 60-day mortality) over time. Clinical characteristics and outcomes were summarized by study date, grouped as: 1) 1984-1990, 2) 1991-1995, 3) 1996-2000, 4) 2001-2005, 5) 2006-2009. Two multivariable logistic regression modeling approaches were used to estimate the ORR and treatment-related mortality rates with A) a comparison of subgroups with 1984-1990 as the reference group, and B) time as a continuous variable.
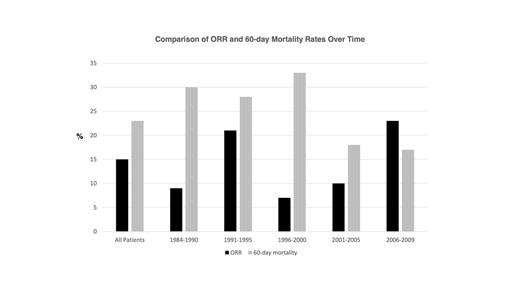
Results: The number of AML patients enrolling on phase 1 clinical trials increased substantially over time (1984-1990: n=61, 2006-2009: n=256). The clinical characteristics resembled a high-risk, relapsed/refractory patient population: median age = 60 years, median # of prior therapies = 4 (63% had ≥3 prior therapies), and median white blood cell count = 3,600/mm3. The proportion of patients ≥60 years increased from the earliest subgroup (1984-1990: 18%) to the most recent (2006-2009: 64%). Additionally, there was a relative increase in the proportion of patients without any prior therapies over time from 1984-1990 (13%) to 2006-2009 (24%). Figure 1 demonstrates the ORR and treatment-related mortality for each subgroup. The ORR for the entire cohort was 15%. ORR was significantly improved in the most recent subgroup (2006-2009: ORR=23%) when compared with the earliest subgroup (1984-1990: ORR=9%), p=0.05. Additionally, there was a small but significant improvement in ORR and 60-day mortality rates with each increasing year, odds ratio (OR)=1.04, p=0.03, OR=0.96, p=0.008, respectively.
Discussion: Our analysis of CTEP-sponsored phase 1 clinical trials demonstrates a relative upsurge of AML patients being enrolled in phase 1 studies over the last 26 years. ORRs and 60-day mortality rates appear to be modestly improving over time, which may be due to the relative increase of treatment-naïve patients enrolling in phase 1 studies. To our knowledge, this is the largest compilation of phase 1 clinical trials reported for AML. Continued enrollment of AML patients on early-phase clinical trials at all stages of disease, including those with newly diagnosed AML with poor-risk features, is vital for drug development and improvement in therapeutic outcomes.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal