Abstract
Background
Conventional induction therapy of acute myeloid leukemia (AML) is still largely based on the combination of cytarabine (ARA-C) and daunorubicin. In the last two decades alternative drug combinations have been tested in order to improve complete remission (CR) rate and quality of remission. Fludarabine has been shown to enhance ara-CTP accumulation in leukemic blasts and to inhibit DNA repair mechanisms, thus providing a rationale for combination with DNA damaging agents. In our institution a fludarabine containing induction regimen (FLAI-5) is being used since more than 15 years.
Patients and methods
Eighty-four consecutive non M3 AML patients (age 17-72 years) treated in our center between 2006 and 2013 were retrospectively analyzed. Median follow-up was 42 months.
Induction regimen included fludarabine 30 mg/sqm and ARA-C 2g/sqm on days 1 to 5 plus idarubicin 10 mg/sqm on days 1-3-5, with or without gemtuzumab ozogamicin (3mg/sqm) on day 6.
Patients achieving complete remission received a second course including ARA-C 2g/sqm on days 1 to 5 and IDA at an increased dose of 12 mg/sqm on days 1-3-5.
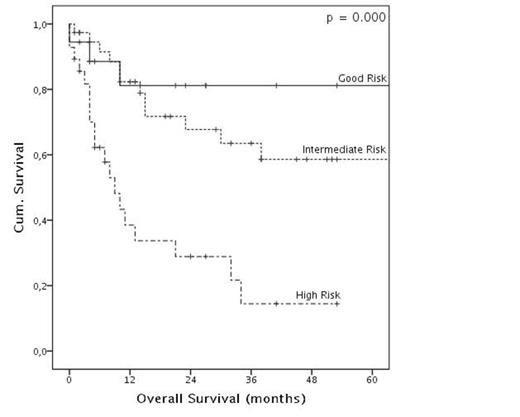
Patients were stratified in prognostic risk groups according to a comprehensive score based on karyotype, de-novo or secondary disease, NPM and FLT3 status. High-risk and selected intermediate-risk patients underwent allogeneic bone marrow transplantation (BMT) in first complete remission if a donor was available. The other patients were scheduled to receive at least 2 and up to 4 courses of consolidation therapy with 4 days of ARA-C (2g/sqm, daily). Patients features, CR rate and overall survival (OS)analysis are summarized in Table 1.
Results and Discussion
Six patients (7%) died during first induction mainly because of infective or hemorrhagic events. Of the remaining 78 patients, 63 patients achieved CR (81%) and 15 did not respond (19%). FLAI-5 was generally well tolerated, with negligible non-hematological toxicity; median time to neutrophil and platelet recovery was 18 and 17 days, respectively. Most patients were able to receive subsequent therapy at full dose and in a timely manner; 33/63 CR patients underwent BMT whereas 21/30 (70%) non-transplanted patients received the full programmed therapy. In the whole cohort, 3 years OS was 50.9% (median 38 months). Factors affecting CR rates and OS are summarized in Tab.1.
In a large randomized trial Burnett et al showed that FLAG-Ida favorably compared with other induction schedule. However the longer disease free survival and the lower relapse rate observed in the FLAG-Ida arm did not translate in a higher OS mostly due to the severe hematological toxicities following the second course.
Our results are comparable to those recently published and show that the administration of fludarabine only in the first course may significantly reduce hematological toxicity therefore allowing patients to proceed along the consolidation program or undergo allo-BMT in a good performance status. Besides, we were able to increase IDA dose (10 and 12mg/sqm in first and second course, respectively, compared to 8 mg/sqm used in the FLAG-Ida combination).
Our results show that FLAI-5 induction regimen followed by a second ARA-C and IDA containing course is an effective and well tolerated therapy for younger AML patients and confirm that CR rate and OS are mainly affected by comprehensive risk group, WBC count at diagnosis and NPM status.
Patients features and CR/OS analysis
| . | . | CR after 1st cycle/Tot. (%) . | p (univ.) . | p (multiv.) . | Median OS (months) . | OS 36 months (%) . | p (univ.) . | P (multiv.) . |
|---|---|---|---|---|---|---|---|---|
| All Patients | 63/78 (81) | - | - | 38 | 50.9 | - | - | |
| Age | < 45 yrs | 28/34 (82) | 1.000 | - | NR | 53.4 | 0.121 | 0.105 |
| > 45 yrs | 35/44 (80) | 15 | 49.2 | |||||
| Karyotype | Favorable | 3/3 (100) | 0.093 | 0.248 | NR | 100 | 0.003 | 0.775 |
| Intermediate | 52/61 (85) | NR | 60.9 | |||||
| Sfavorable | 8/13 (62) | 7 | 0 | |||||
| NPM | Wild type | 36/50 (72) | 0.003 | 0.001 | 32 | 45.1 | 0.270 | 0.541 |
| Mutated | 26/26 (100) | 65 | 66 | |||||
| FLT3 | Wild type | 48/60 (80) | 0.721 | - | 65 | 56.3 | 0.249 | 0.658 |
| FLT3 ITD | 14/16 (88) | 14 | 35 | |||||
| Disease Onset | De novo | 57/67 (85) | 0.031 | 0.213 | 65 | 56.1 | 0.001 | 0.809 |
| Secondary | 6/11 (55) | 7 | 21.4 | |||||
| WBC at diagnosis | <30.000/mmc | 41/47 (87) | 0.087 | 0.020 | 65 | 59.4 | 0.092 | 0.326 |
| >30.000/mmc | 22/31 (71) | 14 | 39.4 | |||||
| Comprehensive Risk Group | Good | 17/17 (100) | 0.009 | 0.983 | NR | 81.2 | 0.000 | 0.001 |
| Intermediate | 31/37 (84) | NR | 63.5 | |||||
| Poor | 15/24 (63) | 9 | 14.4 | |||||
| Mylotarg | FLAI | 44/54 (82) | 1.000 | - | 34 | 49.1 | 0.676 | - |
| MY-FLAi | 19/24 (79) | 65 | 52 | |||||
| . | . | CR after 1st cycle/Tot. (%) . | p (univ.) . | p (multiv.) . | Median OS (months) . | OS 36 months (%) . | p (univ.) . | P (multiv.) . |
|---|---|---|---|---|---|---|---|---|
| All Patients | 63/78 (81) | - | - | 38 | 50.9 | - | - | |
| Age | < 45 yrs | 28/34 (82) | 1.000 | - | NR | 53.4 | 0.121 | 0.105 |
| > 45 yrs | 35/44 (80) | 15 | 49.2 | |||||
| Karyotype | Favorable | 3/3 (100) | 0.093 | 0.248 | NR | 100 | 0.003 | 0.775 |
| Intermediate | 52/61 (85) | NR | 60.9 | |||||
| Sfavorable | 8/13 (62) | 7 | 0 | |||||
| NPM | Wild type | 36/50 (72) | 0.003 | 0.001 | 32 | 45.1 | 0.270 | 0.541 |
| Mutated | 26/26 (100) | 65 | 66 | |||||
| FLT3 | Wild type | 48/60 (80) | 0.721 | - | 65 | 56.3 | 0.249 | 0.658 |
| FLT3 ITD | 14/16 (88) | 14 | 35 | |||||
| Disease Onset | De novo | 57/67 (85) | 0.031 | 0.213 | 65 | 56.1 | 0.001 | 0.809 |
| Secondary | 6/11 (55) | 7 | 21.4 | |||||
| WBC at diagnosis | <30.000/mmc | 41/47 (87) | 0.087 | 0.020 | 65 | 59.4 | 0.092 | 0.326 |
| >30.000/mmc | 22/31 (71) | 14 | 39.4 | |||||
| Comprehensive Risk Group | Good | 17/17 (100) | 0.009 | 0.983 | NR | 81.2 | 0.000 | 0.001 |
| Intermediate | 31/37 (84) | NR | 63.5 | |||||
| Poor | 15/24 (63) | 9 | 14.4 | |||||
| Mylotarg | FLAI | 44/54 (82) | 1.000 | - | 34 | 49.1 | 0.676 | - |
| MY-FLAi | 19/24 (79) | 65 | 52 | |||||
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal