Abstract
Introduction: Clinical trials have demonstrated that the new oral anticoagulants (NOACs), dabigatran, rivaroxaban, and apixaban are either superior or non-inferior to standard therapies/placebo for the treatment of nonvalvular atrial fibrillation (NVAF) and venous thromboembolism (VTE). This study estimated and compared the medical costs from a U.S. payer perspective that may be potentially avoided when NOACs are used instead of standard therapies/placebo for the treatment of patients with NVAF or VTE.
Methods: Event rates of efficacy and safety clinical endpoints as defined in clinical trials (Table), were obtained from published trial data. Medical cost avoidances associated with NOAC usage vs. standard therapies/placebo for NVAF patients, acute VTE treatment patients, and extended VTE treatment patients were derived from previous publications. All costs were inflation adjusted to 2013 cost level. A hypothetical health plan population with 1 million members in 2014 was used to estimate and compare the NVAF and VTE combined medical cost avoidances for patients treated with NOACs vs. standard therapies/placebo. Prevalence rates of NVAF and VTE were derived from published literature. The same usage rate for each NOAC was assumed for comparison purpose. The medical cost avoidances are also projected in the years 2015-2018 and compared among the NOACs.
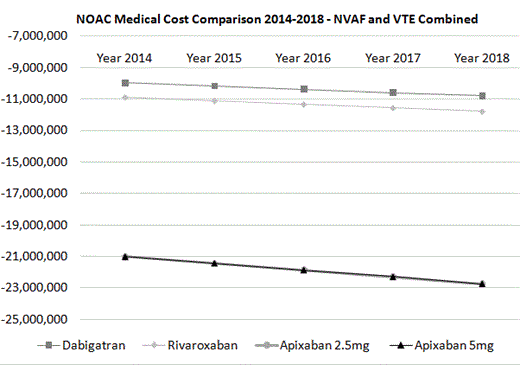
Results: In 2014, in the hypothetical population of one million insured lives, the medical costs were projected to be reduced by -$3.0, -$2.1, and -$7.3 million for NVAF patients treated with dabigatran, rivaroxaban, and apixaban, respectively; by -$0.7, -$2.2, and -$4.1 million for patients treated for acute VTE with dabigatran, rivaroxaban, and apixaban, respectively; and by -$6.3, -$6.6, -$9.6, and -$9.5 million for VTE patients treated for extended periods with dabigatran, rivaroxaban, 2.5 mg apixaban, and 5.0 mg apixaban, respectively (2 arms with different apixaban dosages were included in extended VTE treatment trial). In 2014, for the combined NVAF and VTE patient populations, within the hypothetical population of one million insured lives, medical costs were projected to be reduced by -$10.0, -$10.9, -$21.0, and -$21.0 million for dabigatran, rivaroxaban, 2.5 mg apixaban, and 5.0 mg apixaban, respectively. In the model, the reductions in medical costs associated with usage of the NOACs were projected to steadily increase in the years 2015 to 2018 (Figure).
Conclusions: Medical costs are reduced, when any of the three NOACs are used instead of standard therapy/placebo for the treatment of NVAF or VTE. Apixaban is associated with the greatest reduction in medical costs, which is driven by medical cost reductions associated with both efficacy and safety endpoints among patients with NVAF or VTE. Further evaluation may be needed to validate these results in the real-world setting.
Clinical Trials from which Clinical Event Rates were Obtained
| Trial . | Drug . | Indication . |
|---|---|---|
| RE-LY | Dabigatran | Nonvalvular atrial fibrillation |
| ROCKET-AF | Rivaroxaban | Nonvalvular atrial fibrillation |
| ARISTOTLE | Apixaban | Nonvalvular atrial fibrillation |
| RE-COVER I | Dabigatran | Acute venous thromboembolism |
| RE-COVER II | Dabigatran | Acute venous thromboembolism |
| EINSTEIN-Pooled | Rivaroxaban | Acute venous thromboembolism |
| AMPLIFY | Apixaban | Acute venous thromboembolism |
| RE-SONATE | Dabigatran | Extended venous thromboembolism |
| EINSTEIN-EXT | Rivaroxaban | Extended venous thromboembolism |
| AMPLIFY-EXT | Apixaban | Extended venous thromboembolism |
| Trial . | Drug . | Indication . |
|---|---|---|
| RE-LY | Dabigatran | Nonvalvular atrial fibrillation |
| ROCKET-AF | Rivaroxaban | Nonvalvular atrial fibrillation |
| ARISTOTLE | Apixaban | Nonvalvular atrial fibrillation |
| RE-COVER I | Dabigatran | Acute venous thromboembolism |
| RE-COVER II | Dabigatran | Acute venous thromboembolism |
| EINSTEIN-Pooled | Rivaroxaban | Acute venous thromboembolism |
| AMPLIFY | Apixaban | Acute venous thromboembolism |
| RE-SONATE | Dabigatran | Extended venous thromboembolism |
| EINSTEIN-EXT | Rivaroxaban | Extended venous thromboembolism |
| AMPLIFY-EXT | Apixaban | Extended venous thromboembolism |
Amin:Bristol-Myers Squibb, Pfizer: Consultancy. Off Label Use: Apixaban for the indication of VTE.. Bruno:Bristol-Myers Squibb: Employment, Equity Ownership. Trocio:Pfizer: Employment, Equity Ownership. Lin:Bristol-Myers Squibb, Pfizer: Consultancy, Research Funding. Lingohr-Smith:Bristol-Myers Squibb, Pfizer: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal