Abstract
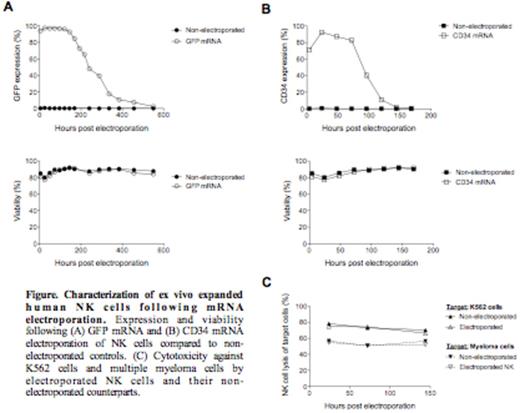
Natural killer (NK) cells are immune cells involved in the defense against cancer. They have also been shown to induce strong anti-tumor responses in the setting of hematopoietic stem cell transplantation and in early clinical trials on adoptive NK cell transfer. Several methods to expand large numbers of clinical-grade NK cells have been developed for trials exploring adoptive NK cell immunotherapy for cancer. However, long-term culturing of NK cells often lead to undesirable phenotypic changes that may compromise their homing capacity and cytotoxic function, and can also lead to senescence compromising in vivo longevity. Introducing genes into NK cells that improve their in vivo viability, cytotoxicity, and ability to home to disease sites could improve the efficacy of NK cell-based immunotherapy. Previously, we and others have shown that genetic manipulation of NK cells through viral transduction is challenging, typically resulting in substantial reduction in NK cell viability and low transduction efficiency. mRNA transfection is an alternative strategy to genetically modify ex vivo expanded NK cells that may overcome limitations of viral transduction. Here we present data characterizing the transgene expression, viability, proliferative capacity, phenotype and cytotoxic function of clinical-grade ex vivo expanded human NK cells following mRNA electroporation using the GMP compliant MaxCyte system. Using unmodified mRNA coding for GFP and the cell surface marker CD34, we established that this technology resulted in rapid and highly efficient protein expression in NK cells without compromising their viability and cytotoxic function (Figure). NK cells electroporated with GFP mRNA rapidly became GFP positive and remained fluorescent for more than two weeks. Following transfection of CD34 mRNA, nearly 100% of NK cells expressed CD34 that remained detectable on the cell surface for up to five days, without affecting viability amongst transfected cells. With the exception of a slight reduction in proliferative capacity compared to controls, no negative impacts of mRNA electroporation using the MaxCyte platform were observed. Transfection of expanded NK cells did not alter expression of twenty cellular markers as assessed by flow cytometry, including activating and inhibitory NK cell receptors and death receptor ligands such as TRAIL. Further, electroporated NK cells maintained high cytotoxic function against K562 cells and multiple myeloma cells (Figure). In conclusion, mRNA electroporation of ex vivo expanded NK cells using the clinical-grade MaxCyte transfection system is highly efficient and opens numerous new possibilities to advancethe field of NK cell-based cancer immunotherapy.
Li:MaxCyte Inc.: Employment, Patents & Royalties. Peshwa:MaxCyte Inc.: Employment, Patents & Royalties; Indian Biomedical Association: Membership on an entity's Board of Directors or advisory committees; Epidarex Capital: Membership on an entity's Board of Directors or advisory committees; BioMetrx LLC: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal