Abstract
Introduction
High rates of complete response (CR) have previously been demonstrated in KRd-treated NDMM pts in a phase 1/2 trial (trial 1; NCT01029054) and a phase 2 trial that combined KRd with autologous stem cell transplant (ASCT) (trial 2; NCT01816971). Here, we report results of extended follow-up from the 2 trials and correlate response and PFS with GEP performed using the MMprofiler GEP assay, which provides results for the SKY92 signature, 7 virtual karyotyping markers (t[4;14], t[11;14], t[14;16]/t[14;20], gain[1q], del[13q], del[17p], H-MM [virtual gain(9q)]), and 3 clusters (MF, MS, CD2). Previously, a combination of 5 markers (SKY92, virtual gain[1q], virtual t[14;16]/t[14;20], MF and CD2 clusters) was identified that predicts improved outcomes when treated with the proteasome inhibitor (PI) bortezomib (van Vliet et al, EHA 2014). We evaluated the SKY92 prognostic signature, virtual karyotyping markers, and this 5-marker PI predictor signature in order to confirm these markers as predictive signatures in the KRd setting.
Materials and Methods
In the consecutive trials 1 and 2, pts received 4 cycles of KRd induction, followed by extended KRd treatment with deferred ASCT (trial 1; NCT01029054) or ASCT followed by extended KRd treatment (trial 2; NCT01816971). In both trials, pts received single-agent lenalidomide as maintenance after completion of KRd. The MMprofiler GEP assay was performed on RNA from CD138+ purified plasma cells. As depth of response with KRd is associated with improved time-to-event outcomes (Jasielec et al, ASH 2013), data were analyzed for associations between any of the MMprofiler markers and the groups that achieved ≥near CR (nCR) vs <nCR by the end of 4 cycles (Fisher exact test). For progression-free survival (PFS), Kaplan-Meier curves were constructed, median survival estimates were calculated, and the Cox proportional hazards model was used to calculate hazard ratios (HR) and associated p-values.
Results
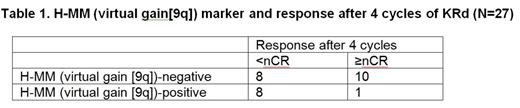
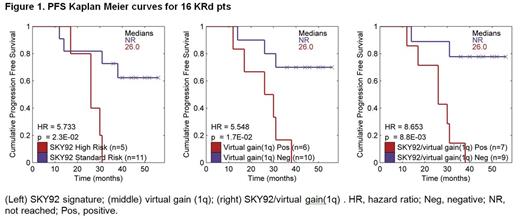
Based on a July 1, 2014 cutoff date, 91 pts were enrolled (53 in trial 1; 38 in trial 2). CD138+ RNA was available from 27 pts (16 from trial 1; 11 from pts in trial 2 who completed ≥4 KRd cycles). Pts with H-MM (virtual gain[9q]) were significantly less likely to achieve nCR (p=0.027) (Table 1). None of the other markers were found to have a statistically significant association with response; several markers, however, showed a trend for predictive value at the current sample size. PFS data were available for 16 pts (median follow-up: trial 1, 40 months [mo]; trial 2, 6.7 mo [with no events at the cutoff date]). Two markers were found to be significantly prognostic (Figure 1): SKY92 (5/16 pts [31%]; HR=5.7; p=0.023) and virtual gain (1q) (6/16 pts [38%]; HR=5.5; p=0.017). Virtual t(4;14) was marginally significant (HR=3.7, p=0.055). When considering the 5-marker PI predictor signature, none of the 16 pts were positive for virtual t(14;16)/t(14;20), MF or CD2 clusters. Combining the SKY92 and virtual gain(1q) markers identified a highly significant subgroup of high-risk pts (7/16; 44%) with a median PFS of 26 mo (HR=8.7; p=0.0088; Figure 1). In no-risk pts (9/16), estimated 4-year PFS was 78%.
Conclusions
Absence of H-MM (virtual gain[9q]) identified pts more likely to achieve ≥nCR by the end of 4 cycles of KRd, with other markers awaiting validation from ongoing enrollment to the KRd + ASCT trial. Based on combined SKY92 high-risk and virtual gain(1q) signatures, the MMprofiler assay detected a highly significant group of high-risk pts with inferior PFS compared to the no-risk group. Despite the inferior outcome, these high-risk pts appeared to have benefited from KRd when compared to historical data of treatment without PIs, suggesting that the 5-marker PI predictor signature (Van Vliet et al, EHA 2014) may also apply to carfilzomib-based treatment. We observed a 4-year PFS rate in no-risk pts in the KRd trials which trended higher than the historical outcome of the PAD arm in the HOVON65/GMMG-HD4 trial (78% vs 34%, respectively), suggesting a benefit for KRd without ASCT in this group of pts compared with other strategies with PIs; further study is needed, however. With ongoing enrollment into the KRd + ASCT trial, the findings reported here will be further validated. Updated results will be presented at the meeting.
Acknowledgments
This research was performed within the framework of CTMM, the Center for Translational Molecular Medicine, project BioCHIP grant 03O-102.
van Vliet:SkylineDx: Employment. Vij:Celgene: Honoraria, Research Funding; Onyx: Honoraria, Research Funding; Sanofi: Honoraria; Janssen: Honoraria; Novartis: Honoraria; Millennium: Honoraria; Array: Honoraria. Dumee:SkylineDx: Employment. Bosman:SkylineDx: Employment. deBest:SkylineDx: Employment. van Beers:SkylineDx: Employment. Jakubowiak:Bristol-Myers Squibb: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Millennium: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Onyx: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; SkylineDX: Honoraria, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal